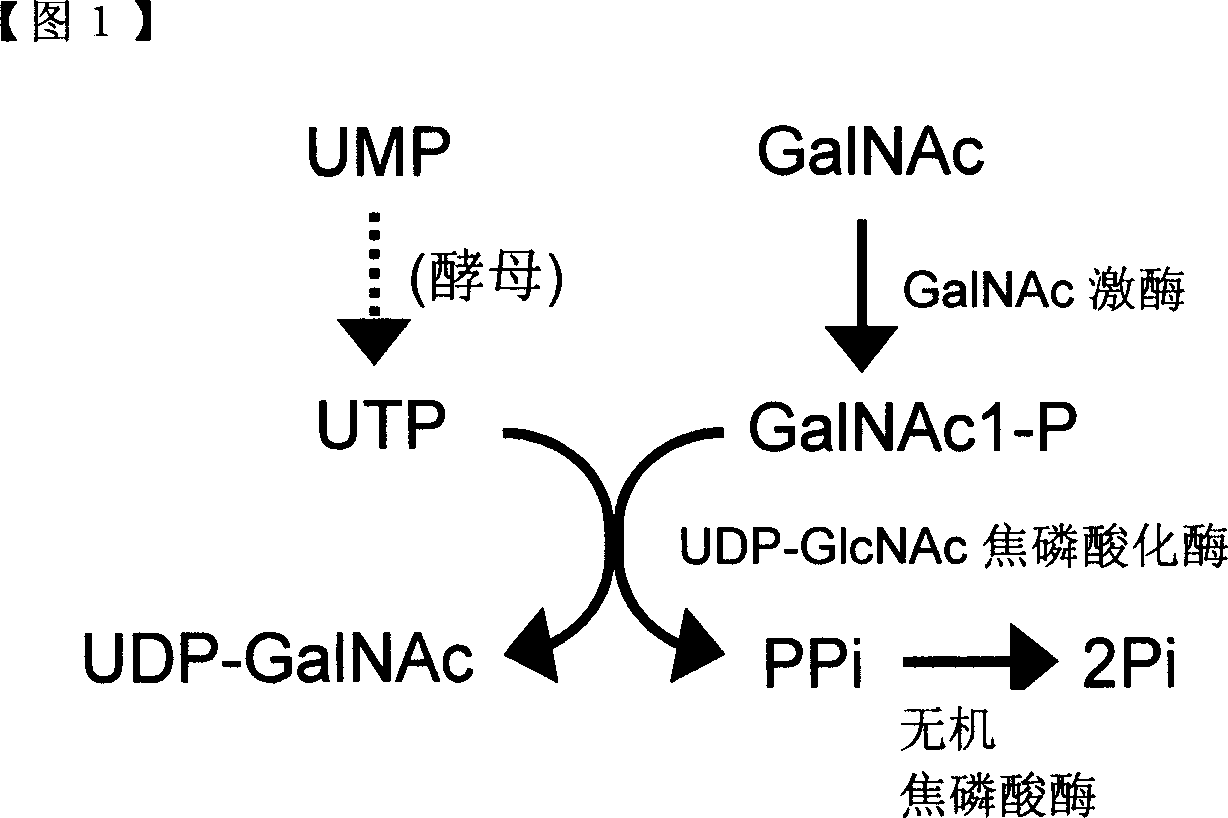
Method of producing uridine 5'-diphospho-N-acetylgalactosamine
The technology of acetylgalactosamine and acetylgalactose is applied in the field of preparation of uridine 5'-diphosphate-N-acetylgalactosamine, which can solve the problems of unpractical method, difficult separation, difficult preparation of enzymes, etc. Easy effect of enzyme preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) Cloning of the GALK 2 gene encoding N-acetylgalactosamine kinase derived from human kidney
[0076] Using the cDNA library derived from human kidney (obtainable from Crontec Company) as a template, the two primer DNAs shown below were synthesized according to conventional methods, and the human kidney GALK 2 gene (submitted to the National Center for Biotechnology Information ( NCBI), the accession number is NM_002044, (Submitted to NCBI, Accession No.NM_002044)) amplification.
[0077] Primer (A): 5'-CGGGGATCCATGGCTACAGAGAGCCCTGCT-3'
[0078] Primer (B): 5'-TACGTCGACTTAGGCCTCAAGCAAAACCAA-3'
[0079] When the GALK 2 gene was amplified by the PCR method, 100 μl of the reaction solution (50 mM potassium chloride, 10 mM Tris hydrochloric acid (pH 8.3), 1.5 mM magnesium chloride, 0.001% gelatin, 0.2 mM dATP, 0.2mM dGTP, 0.2mM dCTP, 0.2mM dTTP, template DNA 0.1ng, primer DNA (A) and (B) each 0.2μM, ExTaq DNA polymerase 2.5U) were heat denatured (94°C, 1 minute), The s...
Embodiment 2
[0096] (1) Synthesis of UDP-GalNAc with human N-acetylgalactosamine kinase and Escherichia coli UDP-GlcNAc pyrophosphorylase
[0097] In 0.2ml solution containing 100mM Tris hydrochloric acid buffer solution (pH7.5), 10mM magnesium chloride, 5mM 5'-UTP·3Na, 5mM GalNAc, 5mM 5'-ATP·3Na, 5mM sodium fluoride, add The obtained N-acetylgalactosamine kinase enzyme solution (0.094 U), UDP-GlcNAc pyrophosphorylase enzyme solution (4.4 U) and inorganic pyrophosphatase (manufactured by Sigma, 0.5 U) at a specified activity amount were kept at 37° C. react.
[0098] During the reaction, an appropriate amount was taken from the reaction liquid, and after boiling for 5 minutes, centrifugation was carried out, and the supernatant was subjected to HPLC analysis. Analysis of the reaction solution 4 hours after the start of the reaction confirmed that 3.2 mM of UDP-GalNAc was synthesized in the presence of N-acetylgalactosamine kinase, UDP-GlcNAc pyrophosphorylase, and inorganic pyrophosphoryl...
Embodiment 3
[0100] (1) Synthesis of UDP-GalNAc using dry yeast and human N-acetylgalactosamine kinase and Escherichia coli UDP-GlcNAc pyrophosphorylase
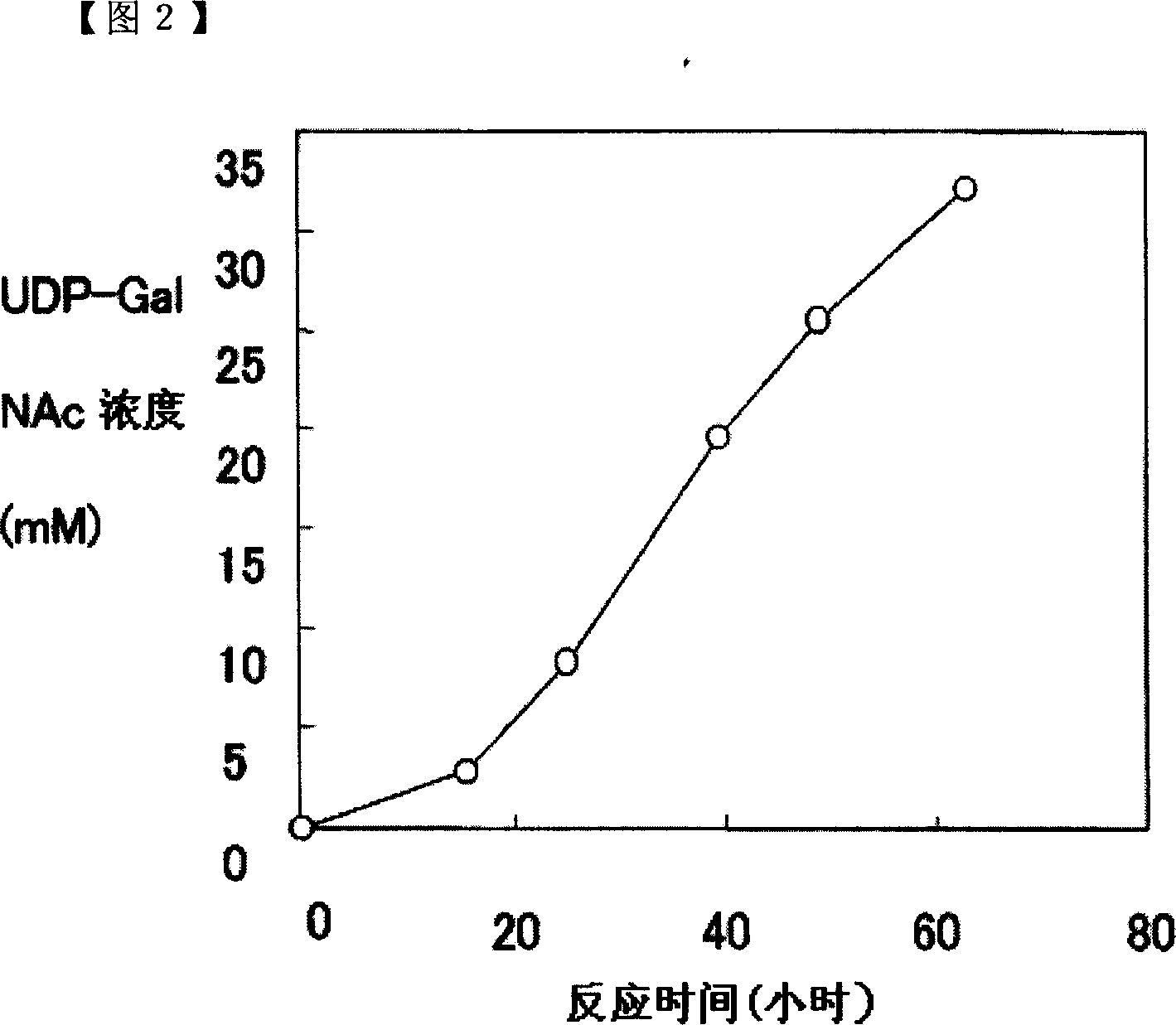
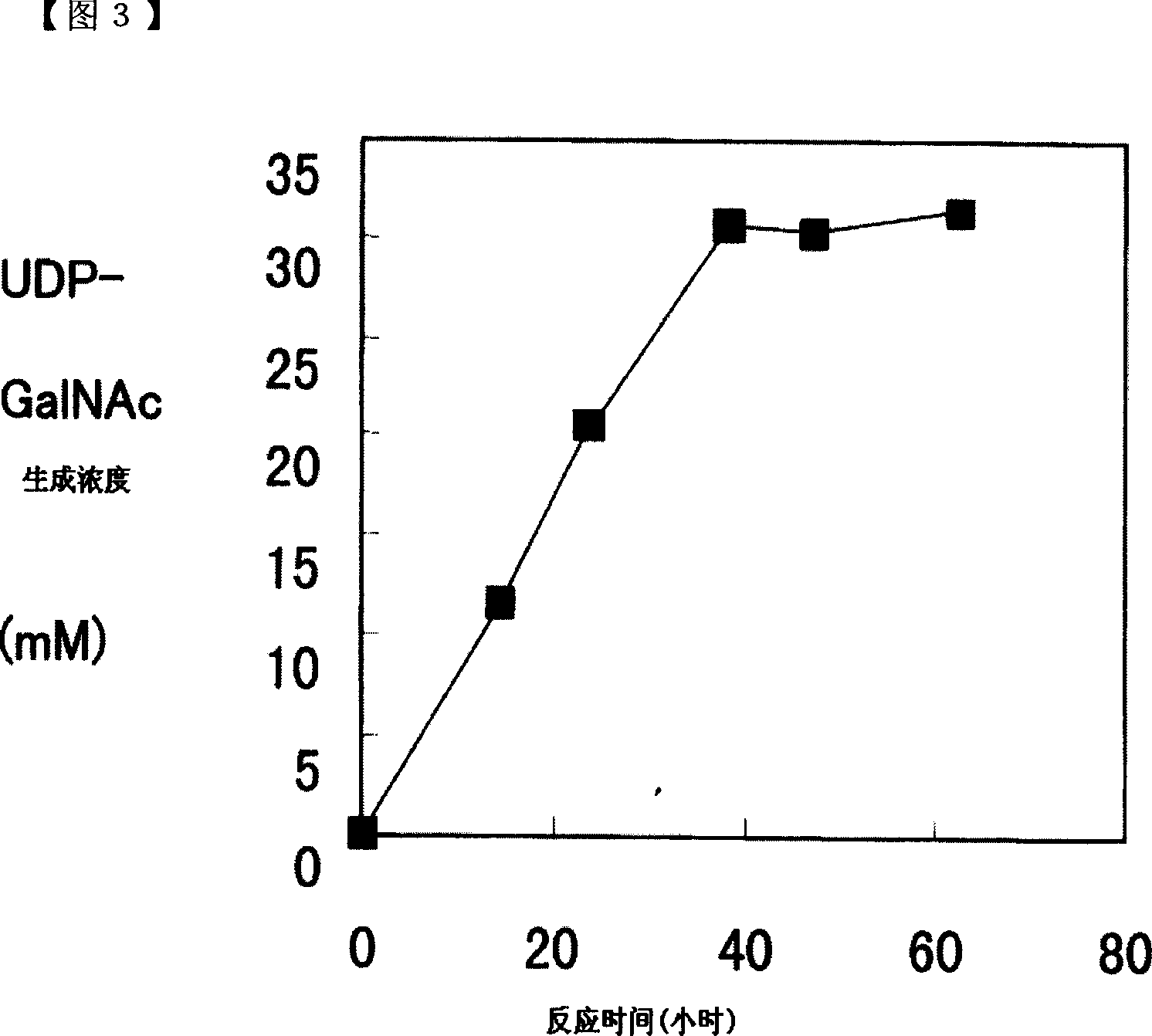
[0101]In 1ml of solution containing 200mM glucose, 50mM GalNAc, 50mM UMP, 200mM potassium phosphate (pH8.0), 20mM magnesium chloride, 3% (w / v) dry baker's yeast (Oriental Yeast Industry), add the mixture prepared in Example 1. Recombinant enzyme (N-acetylgalactosamine kinase; 0.32 U, UDP-GlcNAc pyrophosphorylase; 10.4 U) was reacted at 26° C. while stirring at a stirring speed of 300 rpm. Then, 0.1 ml of a 2M glucose solution was added to the reaction solution at 16, 24, 40, and 48 hours after the start of the reaction. The results of analyzing the reaction solution at different times are shown in FIG. 2 . The accumulated amount of UDP-GalNAc reached 32mM after 49 hours of reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com