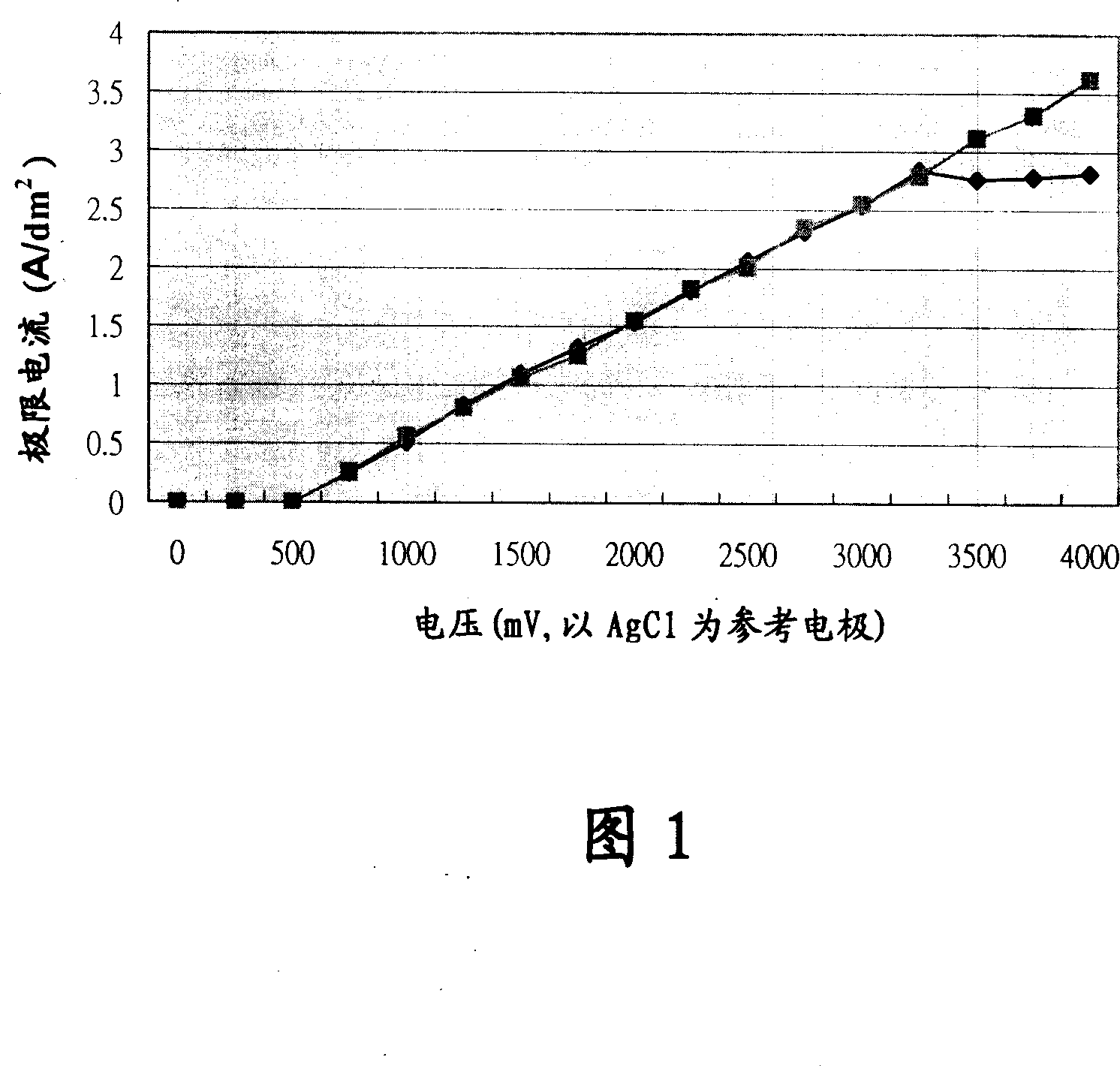
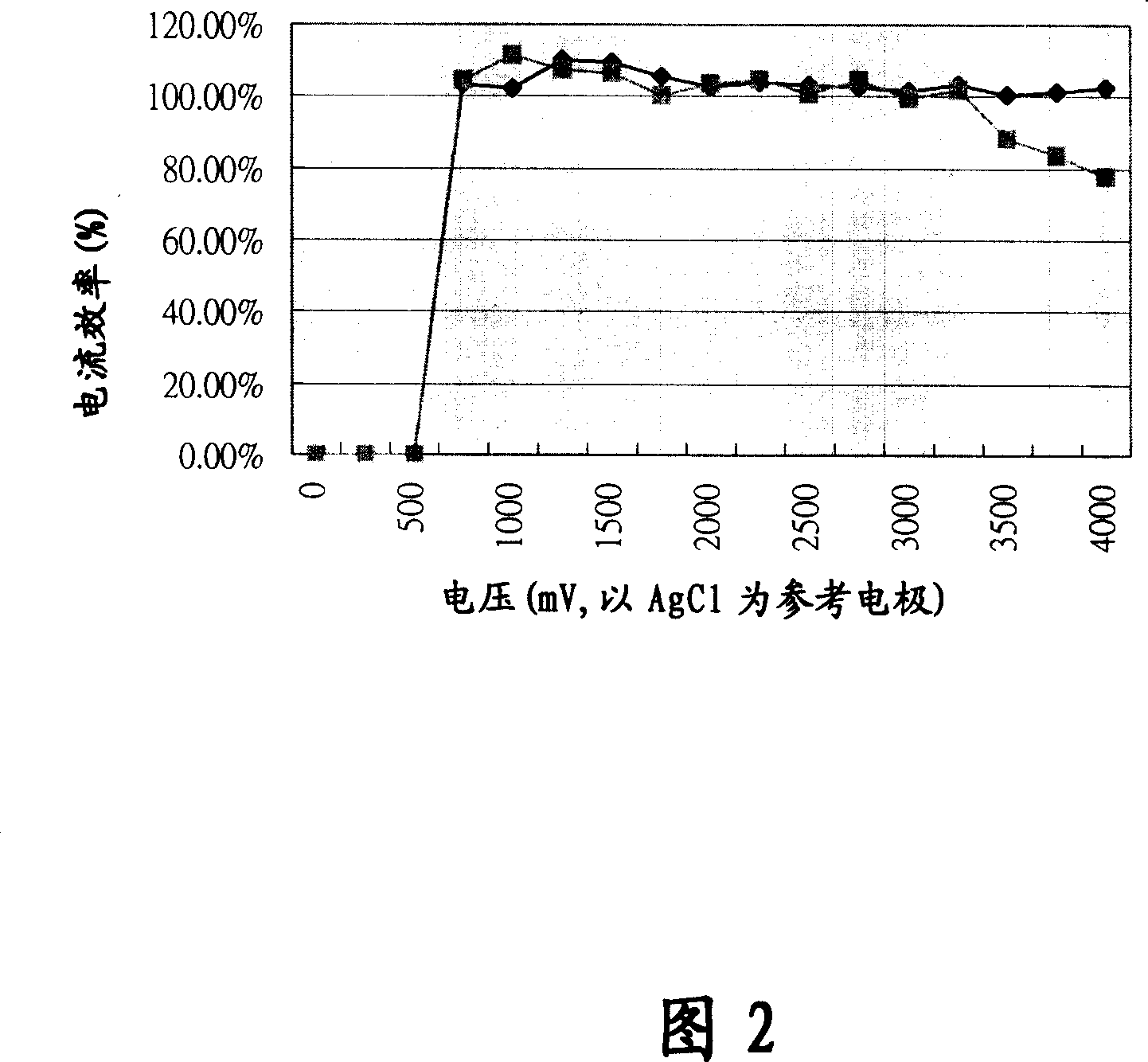
Nickel-cobalt-phosphor electroplating composition, electroplating liquid and electroplating method therewith
A nickel-cobalt-phosphorus and electroplating solution technology is applied to the electroplating composition of nickel-cobalt-phosphorus alloy coating, and the surface treatment of a workpiece can solve the problems of decreased corrosion resistance, cobalt phosphite precipitation, and low reduction ratio, and achieves hardness improvement. Effect
Inactive Publication Date: 2007-11-21
GREAT MAGTECH TECH CO LTD
View PDF2 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The electroplating solution uses sodium hypophosphite as a source of phosphorus content in the coating, and the pH value of the electroplating solution is between 3 and 4.5 during operation, but after the oxidation reaction of hypophosphite (H2PO2-), the vast majority Part of the product is phosphite (HPO32-), and the solubility product constant of phosphite and cobalt ion (whether it is cobalt divalent or trivalent ion) is lower than 10-6 in an environment with a pH value of 4, so It is very easy to cause cobalt phosphite to settle and precipitate in the form of colloid, making the state of the electroplating solution very unstable, which is not conducive to the electroplating operation, so Darell's electroplating formula cannot be applied to stable production operations
The more serious situation is that suspended matter is produced in the electroplating solution, and the electroless plating reaction occurs on the surface of the suspended matter, resulting in collapse
[0006] Secondly, this patent uses sodium hypophosphite as the source of phosphorus content in the coating, and it will also cause sodium ions to release excess hydrogen radicals at the cathode, causing a large amount of hydrogen radicals to penetrate and store in the nickel crystal nucleus, which can easily cause hydrogen in the coating. Undesirable phenomenon of embrittlement
Furthermore, Darell’s electroplating formula uses organic anions as complexes, such as gluconic acid, malic acid, etc., which have a much greater ability to complex nickel ions than cobalt ions, making the polarization of nickel ions much higher than that of cobalt In this way, the cobalt content in the low-current area of the alloy coating is much greater than that in the high-current area, so the alloy coating prepared in this case is prone to internal battery phenomenon, which will cause a sharp drop in corrosion resistance. Upon application After human experiments, it was confirmed that the coating with a thickness of 20 μm prepared by this method can only withstand the salt spray test for 75 hours
In addition, the large difference in the proportion of each metal content in the high and low current areas of the coating will also make the hardness of different areas different, resulting in more serious internal stress problems
[0007] Therefore, it is quite difficult to prepare a nickel-cobalt-phosphorus alloy coating with low internal stress, good hardness, good corrosion resistance and not easy to cause embrittlement. In addition, because the reduction ratio of cobalt ions is very low, the unit price Therefore, if the electroplating solution cannot solve the problem of cobalt ion precipitation and long-term use, it will also cause waste of cobalt raw materials, resulting in high production costs
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0109] This example is prepared by the same steps as Comparative Example 1, except that 90 g of triethylenetetramine is also added in step (a).
Embodiment 2
[0119] Example 2 was prepared by the same steps as Comparative Example 2, except that the electroplating solution of Example 1 was used in step (a). Examples 3 to 7 are prepared by the same steps as Example 2, except that the plating time is increased to 10, 20, 40, 80, and 160 hours, respectively.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
An electroplating composition with nickel, cobalt and phosphor, the electroplating liquid and method are disclosed. The nickel-cobalt-phosphor alloy consists of nickel 68.5-94.5 wt%, cobalt 5-15.5 wt%, phosphor 0.5-16 wt%, phosphite ion No. 1 compound and crossing agent selected from triethenoid tetrammine, triethenoid tri-amino, ethylenediamine and hydro-nitrogen benzide.
Description
Technical field [0001] The invention relates to a nickel-cobalt-phosphorus electroplating composition, in particular to a nickel-cobalt-phosphorus alloy coating that can be used for a long time and can be plated with low internal stress, good hardness, good corrosion resistance and not easy to cause embrittlement. The electroplating composition, the electroplating solution obtained by dissolving it in water, and the method for surface treatment of a workpiece using the electroplating solution. Background technique [0002] Based on various needs, the industry often performs an electroplating treatment on the surface of a substrate, such as: plating precious metals to increase surface gloss; hard chromium (Cr) plating to improve surface hardness and wear resistance; tin plating (Sn) or lead (Pb) to increase corrosion resistance; silver (Ag) or copper (Cu) to increase conductivity, etc. Among them, because the chromium plating layer has the characteristics of high hardness, good co...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C25D3/56
CPCC25D5/50C25D3/562
Inventor 何靖
Owner GREAT MAGTECH TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com