Preparation and application of sage polysaccharides and its esters
A technology of sage and polysaccharide, which can be used in medical preparations containing active ingredients, plant/algae/fungus/moss ingredients, plant raw materials, etc., and can solve the problem of no anti-HIV-1 activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Embodiment 1: A kind of preparation method of compound sage polysaccharide in the present invention
[0010] Salvia yunnanensis C.H.Wright is taken, extracted with hot water, concentrated, ethanol precipitated, deproteinized, and freeze-dried to obtain the salvia polysaccharide of the present invention. The molecular weight measured by molecular sieve chromatography is 26,000 Daltons. The HPLC analysis shows that the monosaccharide composition is sorbitol, galactose, and rhamnose, and the ratio of the three is 5:2:2. The Smith degradation analysis shows that the rat tail There are no 1→2 glycosidic bonds in grass polysaccharides. Further according to the law of periodic acid consumption in polysaccharides, it is deduced that they are connected by 1→6 (or non-reducing ends), 1→4 and 1→3 glycosidic bonds, and the ratio of the three is 1: 3:1. Infrared spectrum analysis showed that at 905cm -1 and 847cm -1 There are absorption peaks on the left and right, which are the ...
Embodiment 2
[0011] Embodiment two: a kind of preparation method of sage polysaccharide sulfate ester in the present invention
[0012] Take 80mL of sulfonating agent and put it in a 250mL three-neck flask, control the temperature at 75°C, add 8 grams of clary sage polysaccharide, stir and react for 3h, take out the flask after the reaction and cool to room temperature, neutralize with 6mol NaOH solution, ethanol precipitation, centrifugation, The precipitate is dissolved in distilled water and dialyzed in distilled water, the dialyzed inner liquid is distilled and concentrated under reduced pressure, precipitated with ethanol, centrifuged, and dried to obtain the sage polysaccharide sulfate of the present invention. The molecular weight measured by molecular sieve chromatography is 18,000 Daltons, and the HPLC analysis shows that the monosaccharide composition is sorbitol, galactose, and rhamnose, and the ratio of the three is 5:2:2, and the degree of sulfation is 1.7. According to the re...
Embodiment 3
[0013] The pharmacological action of clary sage polysaccharide and sulfate ester thereof in embodiment three present invention
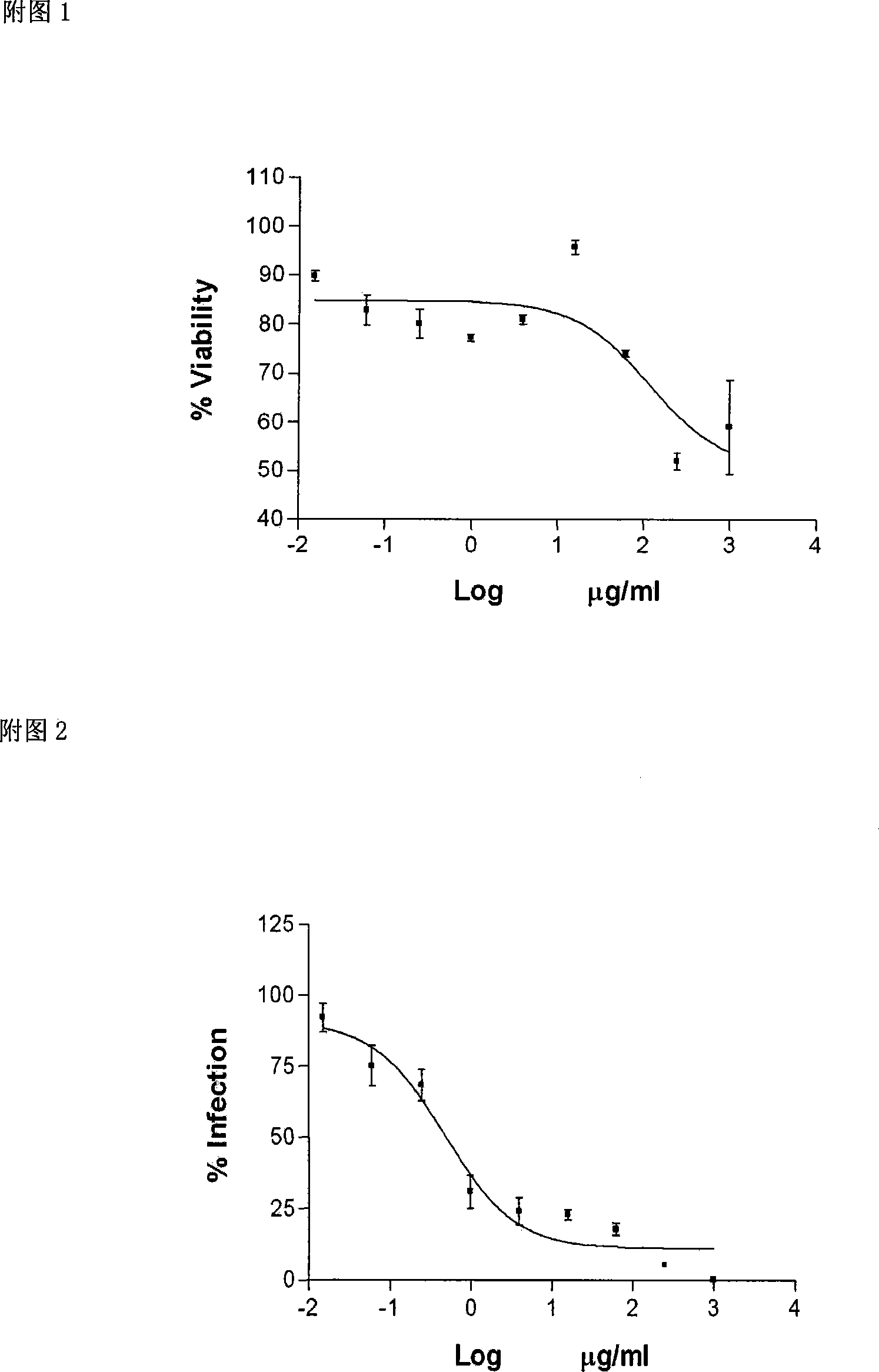
[0014] 1. The cytotoxicity of sage polysaccharide sulfate was determined by MTT method, and the anti-HIV-1Ba-L (R5 virus) activity of sage polysaccharide sulfate was determined by fluorescence measurement technology.
[0015] TZM-b1 cell is a HeLa cell line expressing HIV-1 receptors and co-receptors such as CD4, CXCR4 and CCR5, as well as firefly enzyme expressed under the control of HIV-1 promoter (AIDS Research and References Reagent Program, NIAID, NIH). First, the toxicity of sage polysaccharide sulfate to TZM-b1 cells was determined. The method is as follows: on a 96-well flat-bottomed culture plate, the sage polysaccharide sulfate is diluted serially by 2 times with RPMI-1640 medium. A total of 6 dilutions, 100 μl per well, set up 3 replicate wells, set up a cell control group and a blank control group. Then, add 100 μl containing 3×10 to e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com