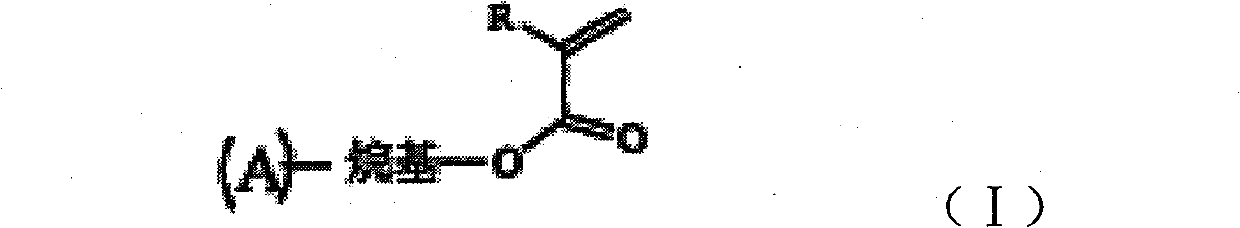
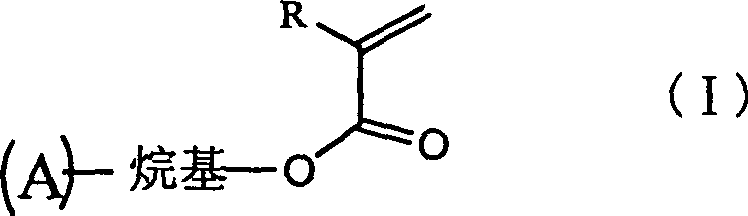
Organic electroluminescence or charge transmission material containing olefine acid ester side group and synthesis thereof
A technology of charge transport and enolate, which is applied in the direction of luminescent materials, electroluminescent light sources, electric light sources, etc., can solve the problems of short circuit, unreported research literature, and impure luminescence, etc., and achieve good solubility, simple reaction, The effect of chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
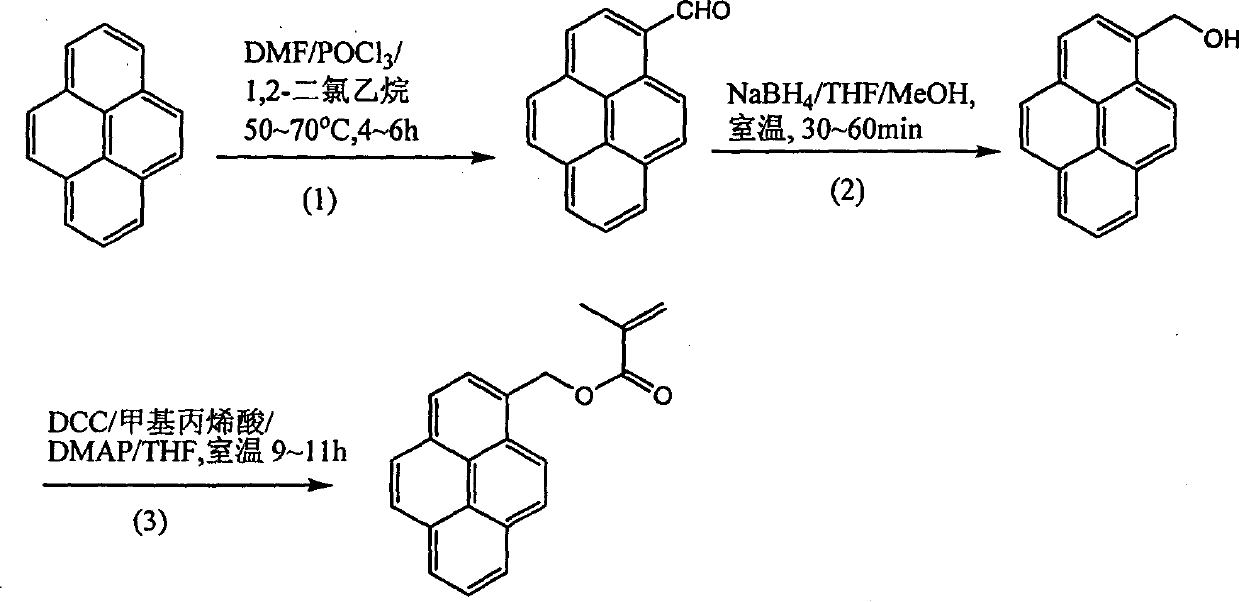
[0016] Embodiment 1: Luminescent material - the synthesis of 2-methacryloyloxymethyl perone and the process of photocross-linking synthesis are as follows:
[0017]
[0018] DMF is dimethylformamide; THF is tetrahydrofuran; MeOH is methanol; DCC is 1,3-dicyclohexylcarbodiimide; DMAP is 4-dimethylaminopyridine.
[0019] The process steps are:
[0020] (1) Synthesis of 2-formyl Piperium from Piperium
[0021] Add POCl to DMF (1.4-1.5g) at 0°C under nitrogen protection 3 (3.0 ~ 3.2g), and then the pernicket (3.9 ~ 4.3g) dissolved in 60ml of 1,2-dichloroethane was added to the reaction system. Heating to 50℃~70℃ for 4~6h. After cooling, 500 ml of water was added to the reaction system, and extracted with ethyl acetate. Na for organic layer 2 CO 3 Aqueous neutralization. After evaporating the solvent, the mixed solvent of toluene-ethyl acetate (volume ratio 9:1) was used as a developing solvent for silica gel chromatography, and the yield was 63%-68%.
[0022] (2) Synthe...
Embodiment 2
[0028] Example 2: The synthesis and photocross-linking process of charge transport material - methacryloyloxymethyl m-TDATA is as follows:
[0029]
[0030] The process steps are:
[0031] (1) Synthesis of aldehyde group m-TDATA
[0032] Add POCl to DMF (1.4-1.5g) at 0°C under nitrogen protection 3 (3.0~3.2g), and then m-TDATA (15.3~16g) dissolved in 250ml 1,2-dichloroethane was added to the reaction system. Heating to 50℃~70℃ for 4~6h. After cooling, 500 ml of water was added to the reaction system, and extracted with ethyl acetate. Na for organic layer 2 CO 3 Aqueous neutralization. After evaporating the solvent, the mixed solvent of toluene-ethyl acetate (volume ratio 9:1) was used as a developing solvent for silica gel chromatography, and the yield was 40%-47%.
[0033] (2) Synthesis of hydroxymethyl m-TDATA
[0034] NaBH was added to 150ml THF solution in which aldehyde group m-TDATA (8.9~9.5g) was dissolved at room temperature 4 (2.0~3.0g) aqueous solution (5...
Embodiment 3
[0039] Example 3: Synthesis and photocrosslinking of luminescent material - poly 9,9'dioctylfluorene-2-methacryloyloxybenzene The synthesis process is as follows:
[0040]
[0041] The process steps are:
[0042] (1) Synthesis of poly 9,9'dioctylfluorene-2-phenol
[0043] Dissolve 5.58g of dioctylfluorene bisboronate, 2.52g of 2,6-dibromophenol and 12-15mg of triphenylphosphorous palladium in 15-20ml of toluene and add 15-20ml of an aqueous solution in which 0.83-0.90g of potassium carbonate is dissolved , React in the dark at 85-90°C for 40-48 hours. After the reaction was completed, the reaction solution was poured into methanol for precipitation, and the precipitate was separated by centrifugation. Dissolve in chloroform, precipitate in methanol, repeat 5 times, and store in dry. Yield 78%~83%.
[0044] (2) Synthesis of poly 9,9'dioctylfluorene-2-hydroxypropoxybenzene
[0045] Add K 2 CO 3 (10.0~12.0g). Stir at 50°C for 12h. Methanol was added to precipitate, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com