Therapeutic HBV DNA vaccines, method for preparing the same and application thereof
A DNA vaccine and vaccine technology, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of DNA shedding, tedious preparation of gold particles, short duration of immune response, etc., and achieve the goal of improving the immune effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Construction of therapeutic HBV DNA vaccine
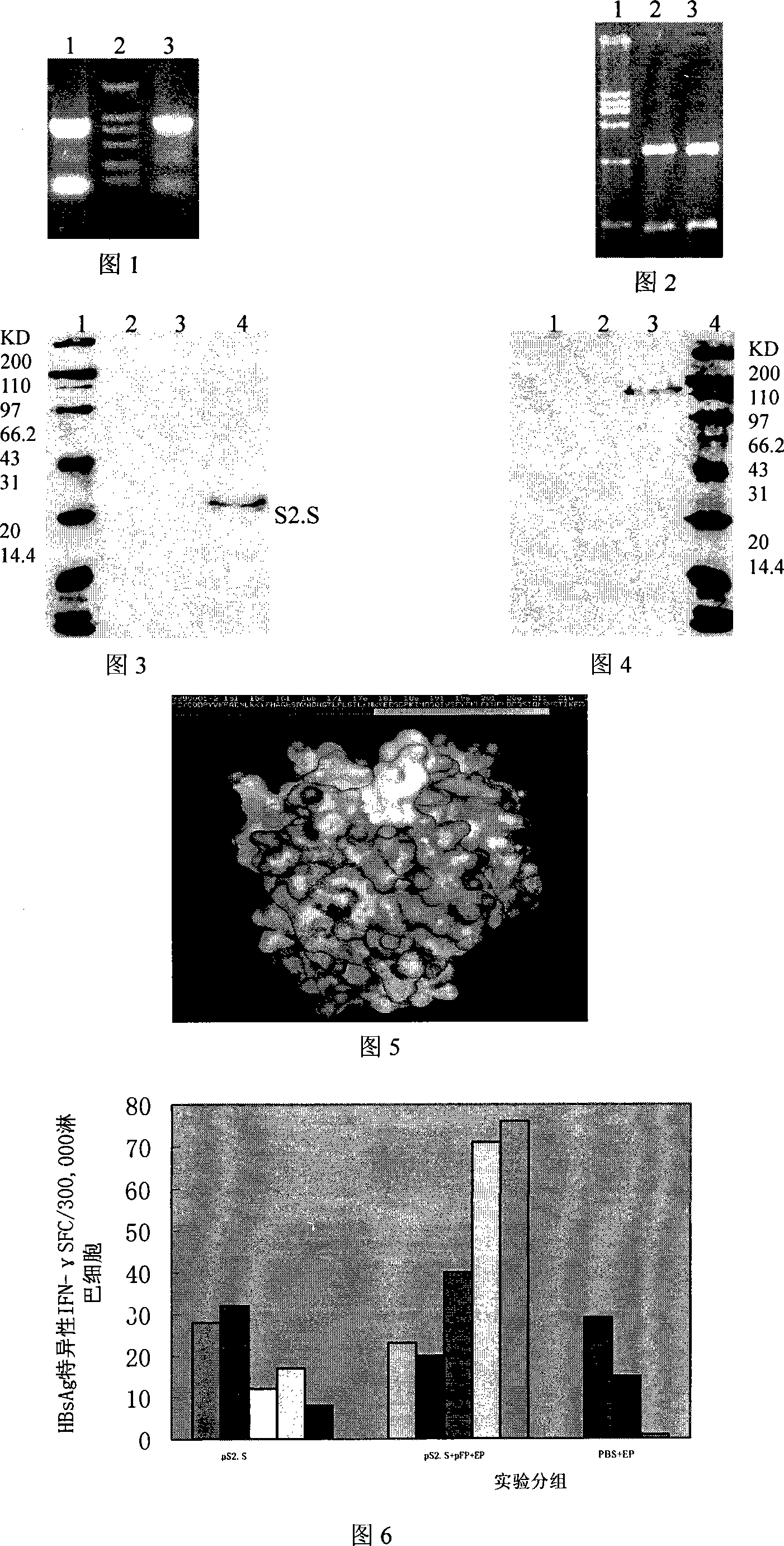
[0063] The therapeutic HBV DNA vaccine includes a DNA vaccine plasmid (abbreviated as pS2.S) whose target gene is the protein preS2+S in the HBV envelope and an adjuvant plasmid (abbreviated as pIIF) whose target gene is human IL2 and IFN-γ.
[0064] We used the eukaryotic expression vector pVAX1 purchased from Invitrogen. According to the requirements of the vector, we added the KOZAK series when designing upstream primers, that is, ANNATGG, -3 is A, +4 is G, to enhance the expression of the target gene. Upstream primer P1 of pS2.S: 5`-GG AAGCT`T AGG ATG GGC TGG CTG CAG AGC CTG-3`, downstream primer P2: 5`-GG GAATT`CTTA AAT GTA TAC CCA AAG ACA A-3', HindIII and EcoRI sites were introduced into the two primers respectively. Using the pcDNA3.1 / preS2.S plasmid as a template, using pfu high-fidelity polymerase, the target fragment was amplified by PCR at 95°C for 5min, 94°C for 50sec, 55°C for 30sec, 72°C for 90sec, 30cycle...
Embodiment 2
[0067] In Vitro Identification of Double Plasmid of Therapeutic HBV DNA Vaccine
[0068] Extract the vaccine plasmid pS2.S and the adjuvant plasmid pIIF according to the instructions of the Endofree Plasmid Mega Kit, and refer to Lipofectamine, the liposome transfection reagent of Invitrogen Company TM 2000 operating instructions are carried out. Cultivate COS-7 cell lines in RPMI1640 medium containing 10% newborn calf serum (FBS) according to conventional methods, digest adherent cells with trypsin 24 hours before transfection, resuspend in serum-containing RPMI1640 medium, press 3× 10 5 Cells / well were transplanted in 6-well culture plate, 37°C, 5% CO 2 After culturing, when the adherent cells grow to 90-95% confluence, take 4 μg of plasmid pS2.S, adjuvant pIIF, pcDNA3.1 / preS2.S plasmid, pcDNA3.1 / hIL2IFNγ plasmid for transfection, and set up untransfected cells at the same time. The transfected cell group and the empty plasmid transfected cell group were used as controls....
Embodiment 3
[0074] Preparation of Double Plasmid of Therapeutic HBV DNA Vaccine
[0075] The preparation process of the HBV DNA vaccine: the preparation method of the vaccine plasmid (pS2.S) and the adjuvant plasmid (pIIF) are the same, but they are produced separately. The fermentation, cracking and purification process are as follows:
[0076] Fermentation: adopt BIOSTAT DL 70 fermenter (Germany B.Braun), with Yeast Extract 8g / L, Tryptone16g / L, NaCL 4g / L, glycerol 24ml / L, NaCl 2 HPO 4 h 2 O 7.08g / L, KH 2 PO 4 12H 2 0 1.58g / L; appropriate amount of trace amino acid as the base medium, with Yeast Extract 100g / L+Tryptone 50g / L+glycerol 400ml / L+MgSO 4 2g / L+an appropriate amount of amino acid is a feed medium fed at a constant rate, with 10% inoculum size, cultured at a constant temperature of 37°C, and the pO2 is controlled below 35%, and the OD600 value of the bacterial density is detected online to calculate the plasmid content. After fermenting for 10 hours, the bacteria were ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com