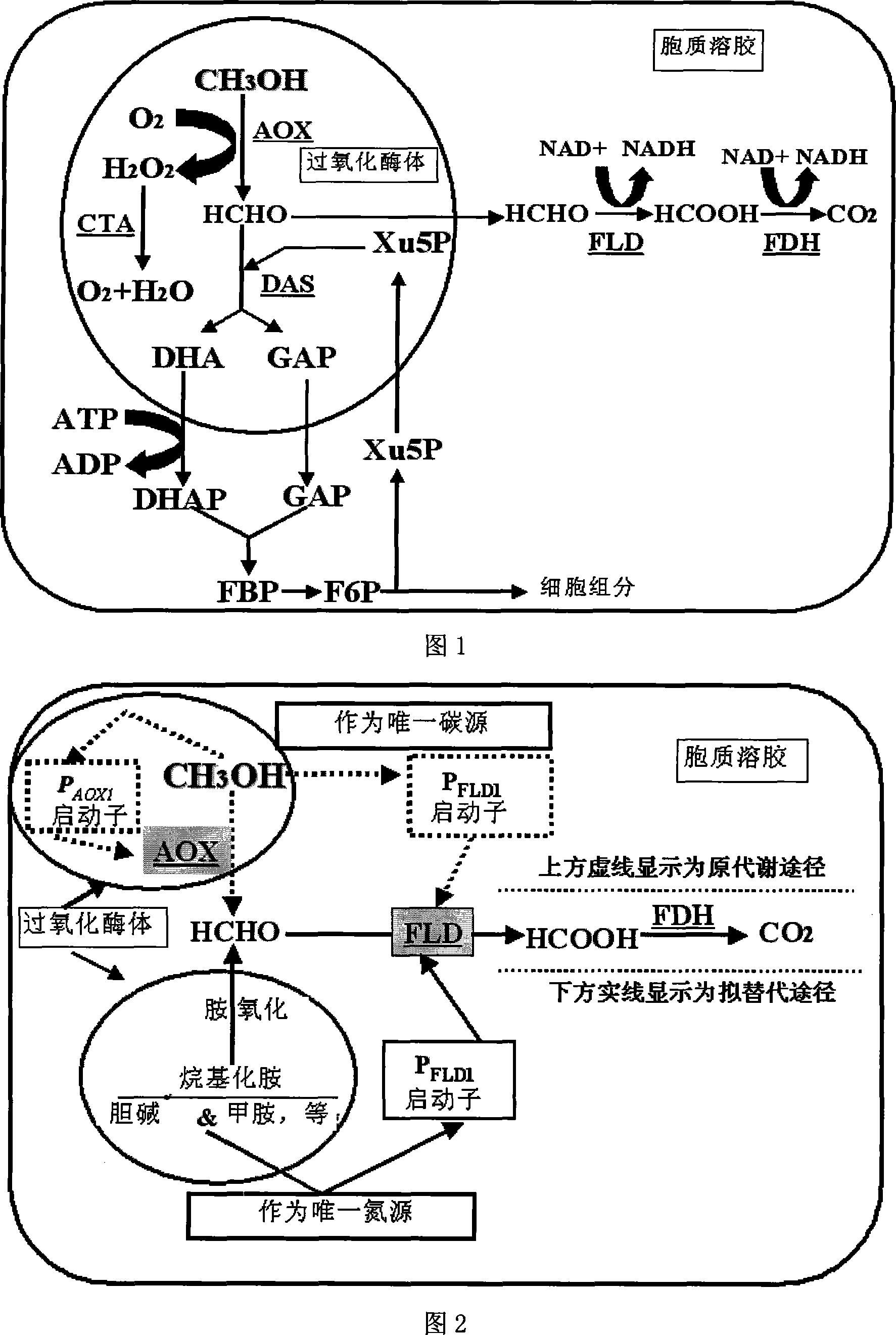
Non-methanol evoked pichia pastoris protein expression system and use thereof
A Pichia pastoris and expression system technology, applied in the biological field, can solve problems such as easy loss of expression plasmids, unstable integration of foreign genes, and unsuitability for high-density fermentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1. Cloning of yeast formaldehyde dehydrogenase protein (FLD1) and its promoter
[0096] Genomic DNA was extracted from yeast cell lines P. pastoris X-33 and P. pastoris GS115, and Saccharomyces cerevisiae using a yeast genome extraction kit. After identification by agarose gel electrophoresis, according to the reported homologous sequences Design and utilize the following pair of primers: PFLD1: 5'-ACG AGATCT GCATGCAGGAATCTCTGGCACG-3 (Bgl II); FLD22: 5'-TCA GGGCCC TTA GTG CAT AGT AAT CAC AGC-3'(Apa I) uses the yeast genome as a template, and uses conventional high-fidelity PCR to amplify a DNA band of about 1.7Kb, which is recovered and ligated with pEGM-T vector to obtain pEGM -T-FLD, after sequence analysis and comparison by Shanghai Yingjun Biotechnology Co., Ltd., it was found that the cloned sequence was shown as SEQ ID NO:1. It is the complete nucleic acid sequence of the yeast formaldehyde dehydrogenase protein (FLD1) including the regulatory regio...
Embodiment 2
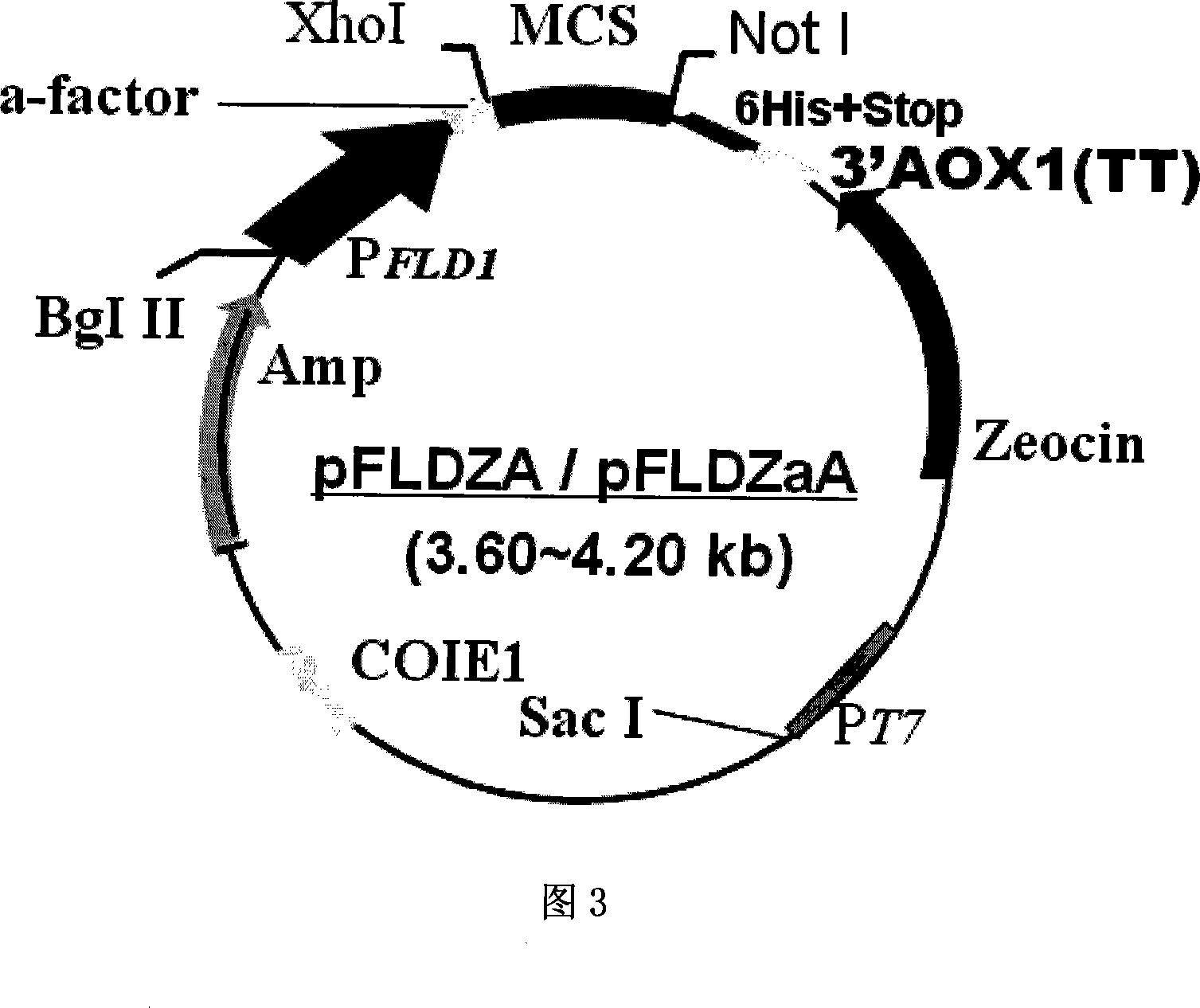
[0097] Example 2. Construction of P.pastoris / pFLDZαA expression system
[0098] In order to construct the secreted expression system of P.pastoris / pFLDZαA with FLD1 as the promoter, we used pEGM-T-FLD as the template and the primer PFLD1:5'-ACG AGA TCT GCA TGC AGG AAT CTC TGGCAC G-3'(Bgl II) and PFLD2a: GCA CAT ATG TGAATATCAAGAATTGTATGAAC-3'(Nde I) amplified the FLD1 promoter sequence of about 600bp with high fidelity, and used pPIC9, pPIC9K, pPIC3.5, pPICZαB, pPICZC, pPICZαC as templates, and Paf1:5'-CGA CAT ATG AGA TTTCCT TCA ATT TTT ACT GCT-3'(Nde I); Paf2: 5'-TCA GGA TCC ACG AGA AGA AACAAA ATG AC-3'(BamH I) amplified about 4000bp contains: yeast secretion signal peptide a- Factor, multiple cloning site region, detection and purification marker region and transcription termination region, the amplification conditions adopt conventional conditions, and finally the previously obtained fragment of about 600bp is combined with one of the previously obtained fragment of ab...
Embodiment 3
[0099] Example 3. Construction of P.pastoris / pFLDZA expression system
[0100] In order to construct the intracellular expression system of P.pastoris / pFLDZA with FLD1 as the promoter, we used pEGM-T-FLD as the template and the primer PFLD1:5'-ACG AGA TCT GCA TGC AGG AAT CTC TGGCAC G-3'(Bgl II) and PFLD2:5'-CTG GGA TCC GAA TTC TGT GAA TAT CAA GAATTG TAT GAA C-3'(BamH I-EcoR I) high-fidelity amplified FLD1 promoter sequence of about 600bp, and then this fragment was combined with about 2500bp recovered from pPICZA cut by Bgl II-EcoR I Contains: Yeast secretion signal peptide a-factor, multiple cloning site region, resistance selection marker, detection and purification marker region connected to the fragment of the transcription termination region, transformed into Escherichia coli DH5a strain, extracted vector digestion, PCR and sequence The determination and identification were all in line with expectations, and finally the pFLDZA expression vector for P. pastoris expres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com