Straight chain alkyl phenol homogeneity polyethenoxy ether acetic acid surfactant and method of preparing the same and use thereof
A straight-chain alkylphenol, polyoxyethylene ether technology, applied in chemical instruments and methods, dissolution, chemical/physical processes, etc., can solve the problem of slow degradation of alkylphenols, unfavorable surfactant applications, product performance differences, etc. problem, to achieve the effect of stable performance and favorable biodegradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
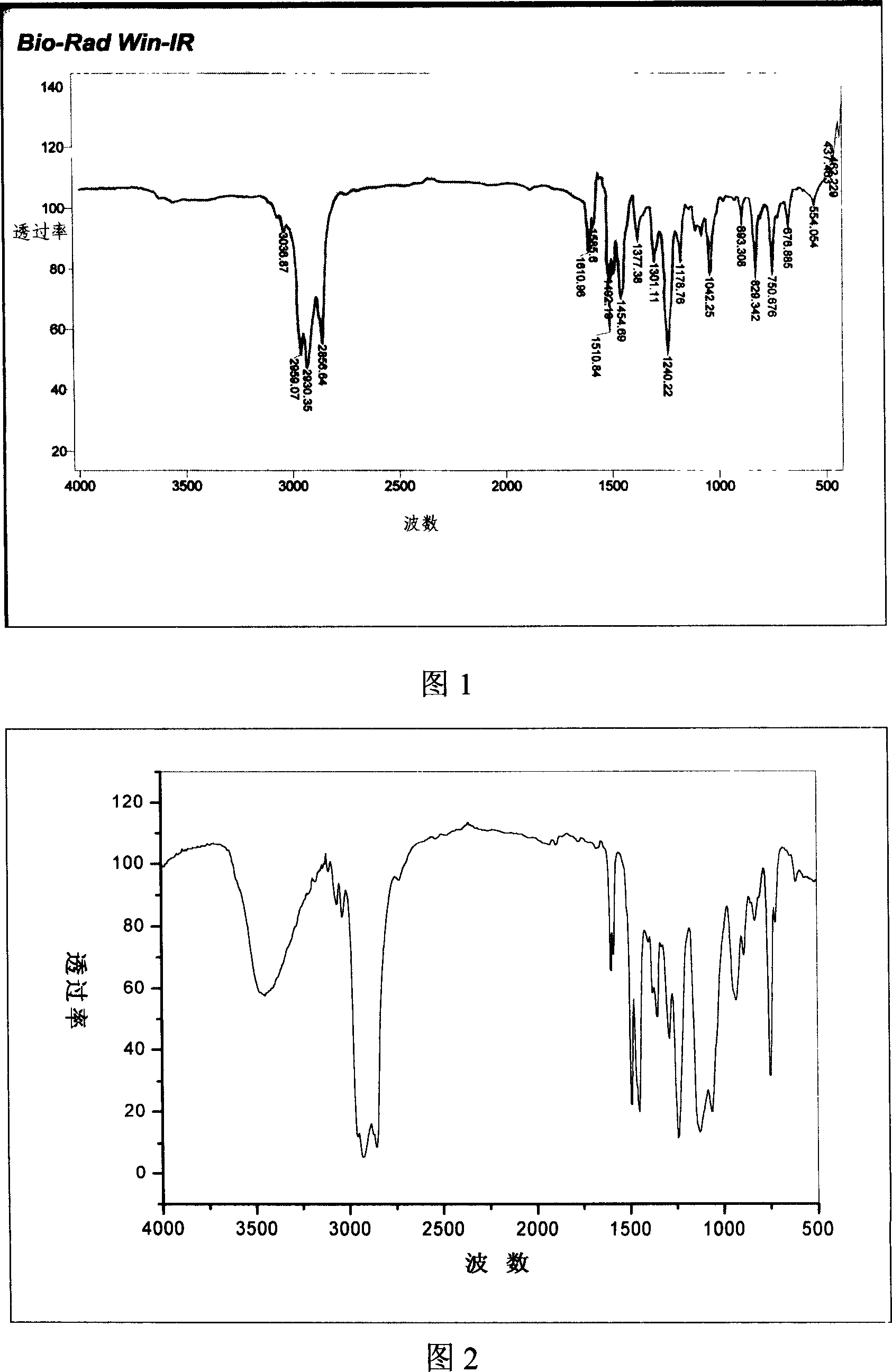
[0073] The synthesis of embodiment 1 laurylphenol trioxyethylene ether sodium acetate
[0074] (a) Synthesis of straight chain lauryl phenol
[0075] method one:
[0076] Add 5 mol of phenol into a three-neck flask equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel, add 5 mL of boron trifluoride ether solution at room temperature, and after mixing thoroughly, add 1 mol α-deca Diene, after the dropwise addition, the reaction temperature was controlled to be 50° C. and maintained for 8 hours. After the reaction was completed, excess phenol was distilled off, and then distilled under reduced pressure to collect high boiling range fractions, which were linear laurylphenols, with a yield of 90%.
[0077] Method Two:
[0078]Add 1 mol of phenol into a three-necked flask equipped with magnetic stirring, raise the temperature to 70°C, add 1.05 mol of lauroyl chloride dropwise, continue the reaction until no gas is released after the addition, s...
Embodiment 2
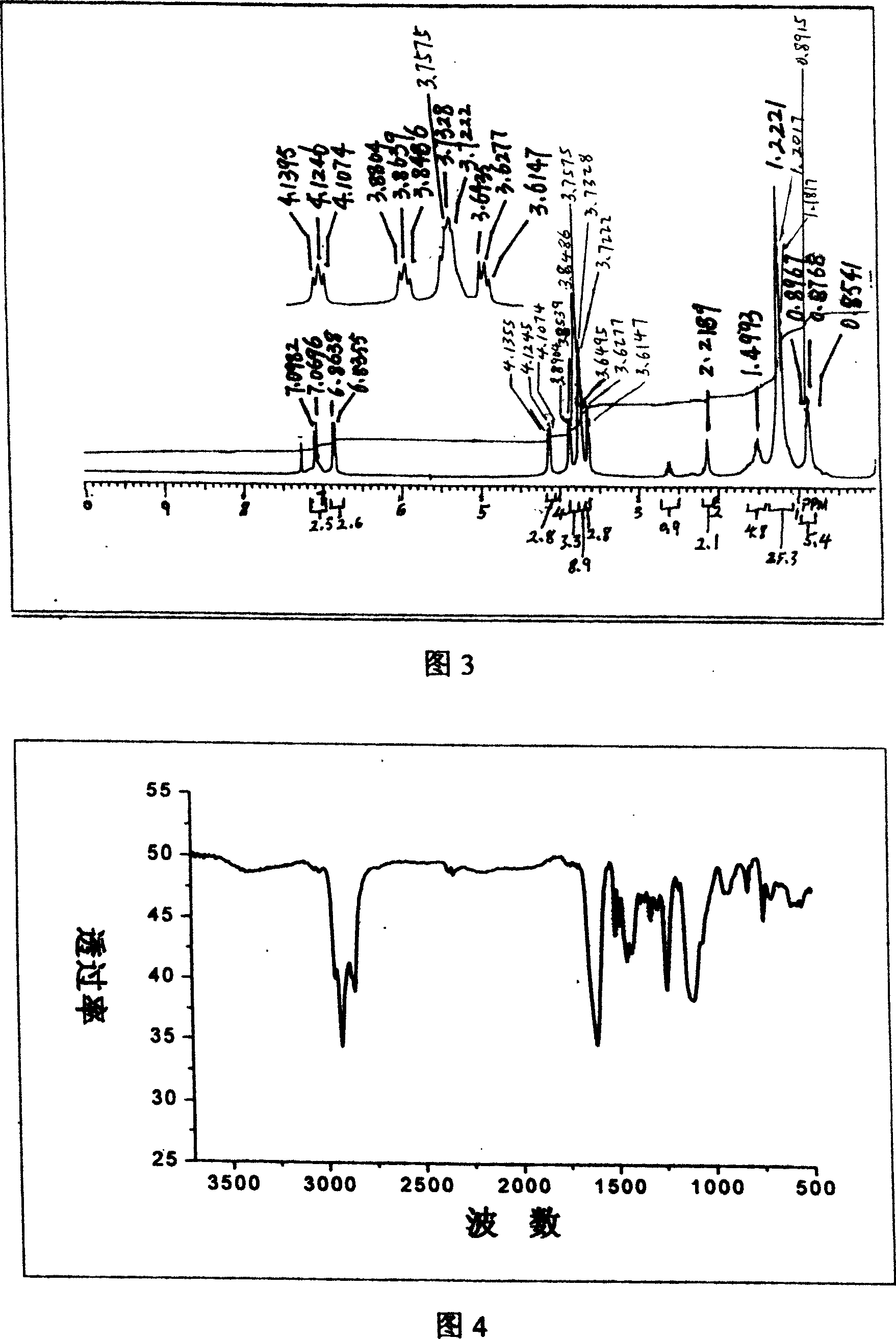
[0087] The synthesis of embodiment 2 laurylphenol trioxyethylene ether ammonium acetate
[0088] (a) Synthesis of straight chain lauryl phenol
[0089] method one:
[0090] Add 5 mol of phenol into a three-neck flask equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel, add 5 mL of boron trifluoride ether solution at room temperature, and after mixing thoroughly, add 1 mol α- Dodecene, after the dropwise addition, the reaction temperature was controlled to be 50° C. and kept for 8 hours. After the reaction was completed, excessive phenol was evaporated, and then vacuum distillation was carried out to collect high boiling range fractions, which were linear laurylphenols, with a yield of 90%.
[0091] Method Two:
[0092] Add 1 mol of phenol into a three-necked flask equipped with magnetic stirring, raise the temperature to 70°C, add 1.05 mol of lauroyl chloride dropwise, continue the reaction until no gas is released after the addition, st...
Embodiment 3
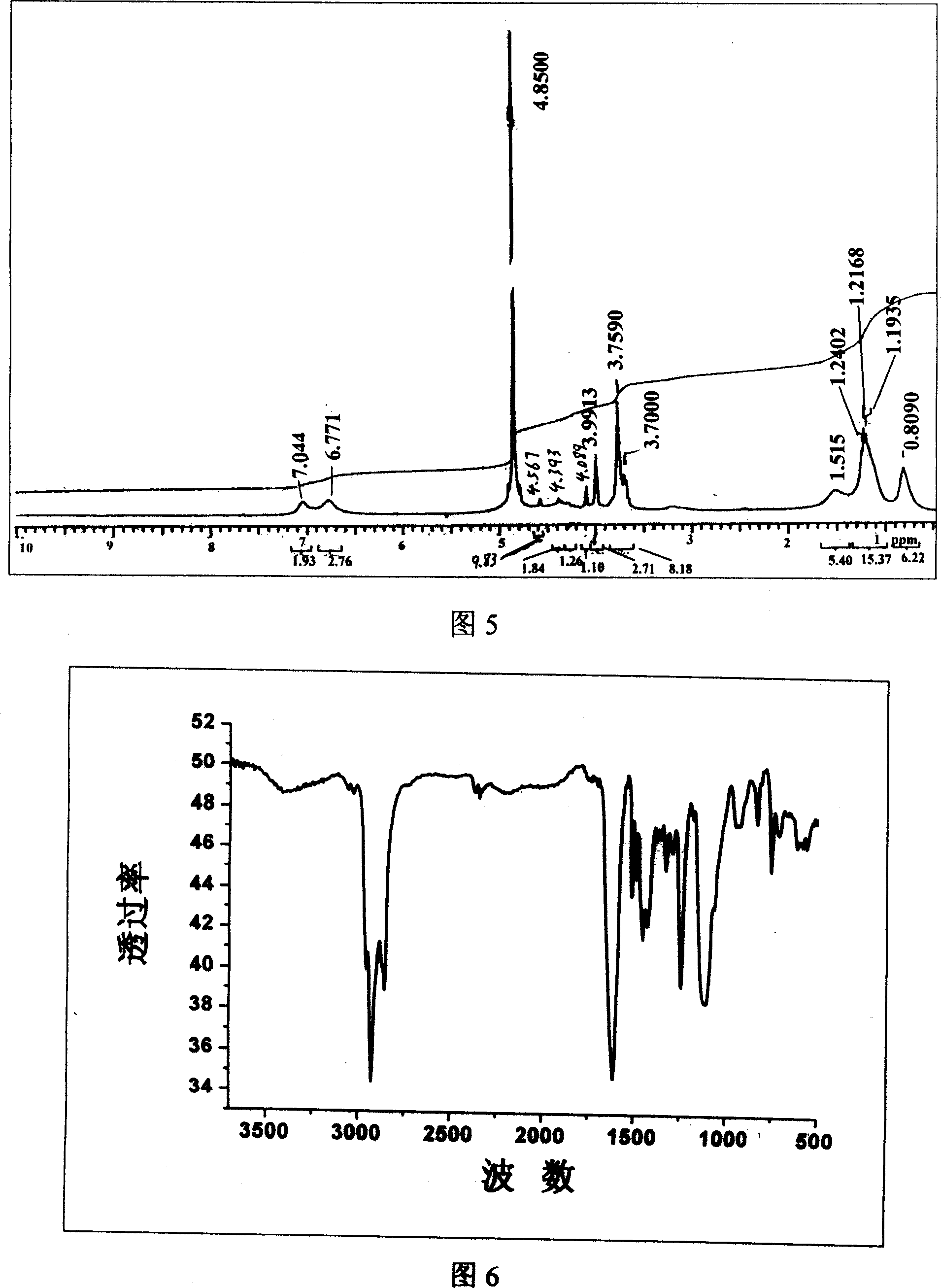
[0101] The synthesis of embodiment 3 octylphenol hexaoxyethylene ether barium acetate
[0102] (a) Synthesis of straight-chain octylphenol
[0103] method one:
[0104] Add 5 mol of phenol into a three-necked flask equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel, add 3 mL of boron trifluoride ether solution at room temperature, and after mixing thoroughly, add 1 mol α- Octene, after the dropwise addition, the reaction temperature was controlled to be 45° C. and kept for 4 hours. Excessive phenol was distilled off after the reaction, and then distilled under reduced pressure to collect high-boiling range fractions, which were linear octylphenols, with a yield of 93%.
[0105] Method Two:
[0106] Add 1 mol of phenol into a three-necked flask equipped with magnetic stirring, raise the temperature to 65°C, and drop in 1.05 mol of octanoyl chloride dropwise. Phenyl octanoate.
[0107] Take 0.8mol of the above-mentioned synthesized phen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com