Novel recombinant parathyroid hormone (1-34) correlated peptide nose drops
A technology of parathyroid hormone and nasal drops, applied in the field of nasal drops of recombinant parathyroid hormone-related peptides, which can solve the problems of weakening activity, stimulating AC biological activity loss, and activity reduction, so as to achieve good absorption and avoid pain , Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 nasal drops
[0032] Pro-Pro-[Arg 11 ]hPTH(1-34)-Pro-Pro 5mg (low), 12.5mg (medium), 25mg (high)
[0033] 2,6-Dimethyl-β-cyclodextrin 400mg
[0034] Polyacrylic acid 25mg
[0035] Citric acid·2H 2 O 84mg
[0036] EDTA 1mg
[0037] Adjust pH to 6.0 with NaOH
[0038] Add water for injection to 10mL
[0039] Get above-mentioned components and prepare Pro-Pro-[Arg 11 ]hPTH(1-34)-Pro-Pro Nasal Drops.
Embodiment 2
[0040] Embodiment 2 nasal drops treat the osteoporosis of ovariectomy rat
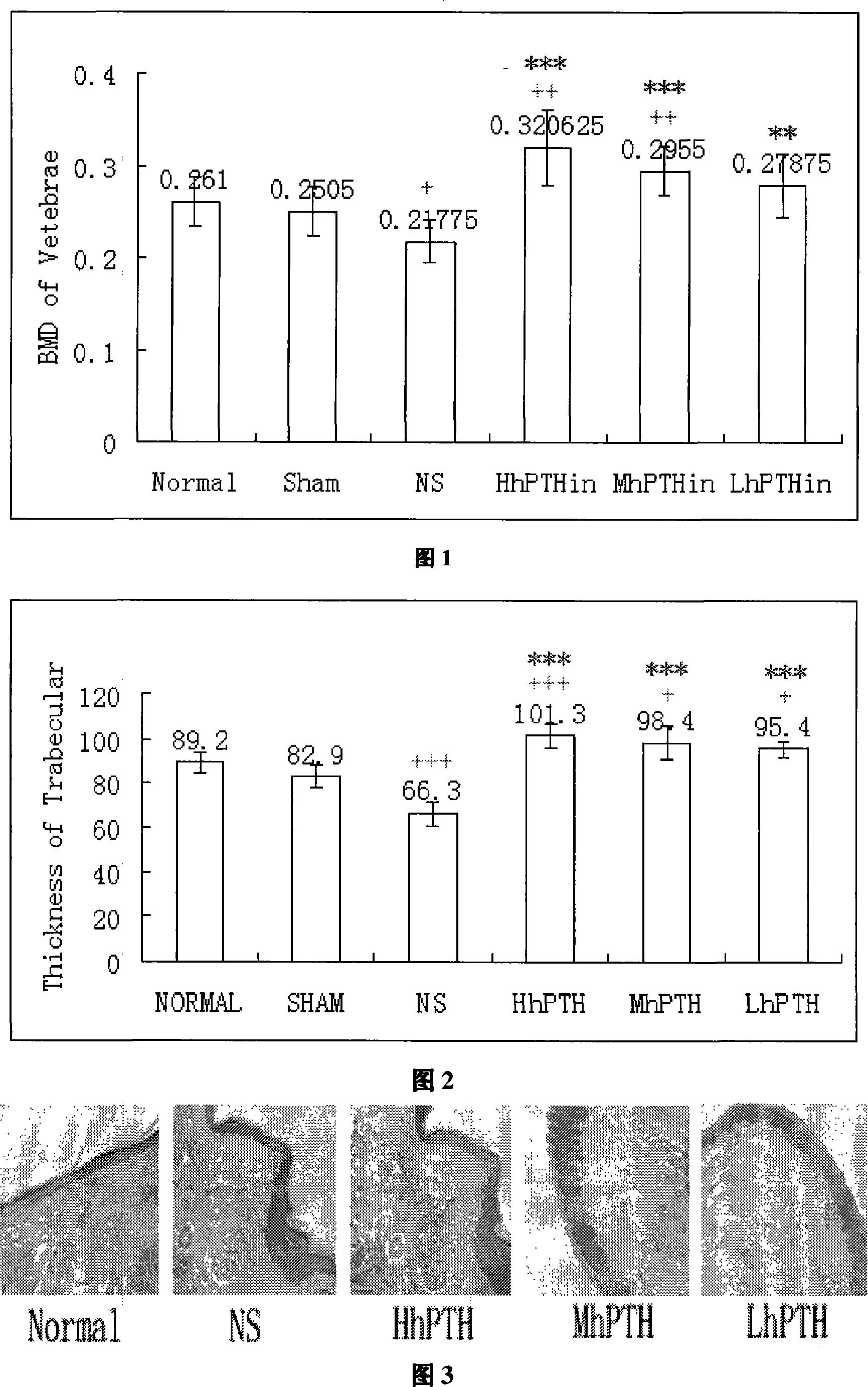
[0041] Divide 48 rats of about 200g into 6 groups according to body weight, which are normal control group (Normal), sham operation group (Sham), normal saline group (NS), and high, medium and low dose groups (HhPTH, MhPTH , LhPTH), all rats except the normal control group were subjected to ovariectomy. After feeding for 2 weeks, the high, medium and low dose groups were instilled with 80 μl of nasal drops of corresponding concentration every day, and the normal saline group was instilled with 80 μl of normal saline. After 12 weeks of continuous administration, the BMD of the femur and vertebrae were measured with a dual-energy X-ray absorptiometry instrument produced by Lunar Corporation of the United States. Vertebral bone density measurement results are shown in Figure 1, and bone bone density measurement results are shown in Figure 2. As can be seen from Fig. 1, compared with normal saline, the h...
Embodiment 3
[0042] The toxicity test of embodiment 3 nasal drops to rat nasal mucosa epithelial cells
[0043] The rats in Example 2 were sacrificed, and the nasal mucosa was taken, fixed with 10% formaldehyde, routinely drawn, embedded in paraffin, made into slices (4 μm thick), stained with HE, and observed with an optical microscope. The results are shown in accompanying drawing 3, as can be seen from the figure that Pro-Pro-[Arg 11 ] hPTH(1-34)-Pro-Pro intranasally administered in each dosage group, the nasal mucosa tissue surface of the rats covered with squamous epithelium is complete, no erosion, ulcer formation, edema and inflammatory cell infiltration in the lamina propria, so it can be concluded Conclusion Pro-Pro-[Arg 11 ] hPTH(1-34)-Pro-Pro nasal drops have no toxicity to rat nasal mucosal epithelial cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com