Recombination human collagen and biological synthesis method thereof
A human-like collagen, biosynthetic technology, applied in the biological field, can solve the problem of not being able to form Hyp residues, achieve high thermal denaturation temperature and thermal decomposition temperature, high thermal stability, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Recombinant human-like collagen containing a non-collagen region at the carboxy-terminal
[0050] The recombinant human-like collagen in this example is composed of a collagen region and a non-collagen region; the non-collagen region is located at the carboxyl terminal of the collagen region, and the collagen region is expressed after 8 repeated polymerizations of the target DNA monomer. The structural features of the designed sequence are: a β-helix-like trimer formed in the non-collagen region, and this trimer with a β-hairpin shape forms a β-sheet, and this region has a strong hydrophobic effect. In the collagen molecule During the assembly process, it can play the role of a node, twisting the peptide chains of the three collagen regions together to form a triple superhelical structure, thereby improving the thermal stability of the recombinant human-like collagen.
[0051] A designed recombinant human-like collagen has the amino acid sequence shown in SEQ...
Embodiment 2
[0105] Example 2: Experimental research on recombinant human-like collagen
[0106] (1) Put a large amount of expressed and purified recombinant humanoid protein into a dialysis bag, put it into distilled water for dialysis for three days, and change the distilled water intermittently at an appropriate time to remove a large amount of salt ions.
[0107] (2) Properly concentrate the protein solution after dialysis, put it into a -80°C refrigerator for pre-cooling for more than 2 hours, put it into a freeze dryer, and freeze-dry it for 20-24 hours to obtain recombinant human-like collagen.
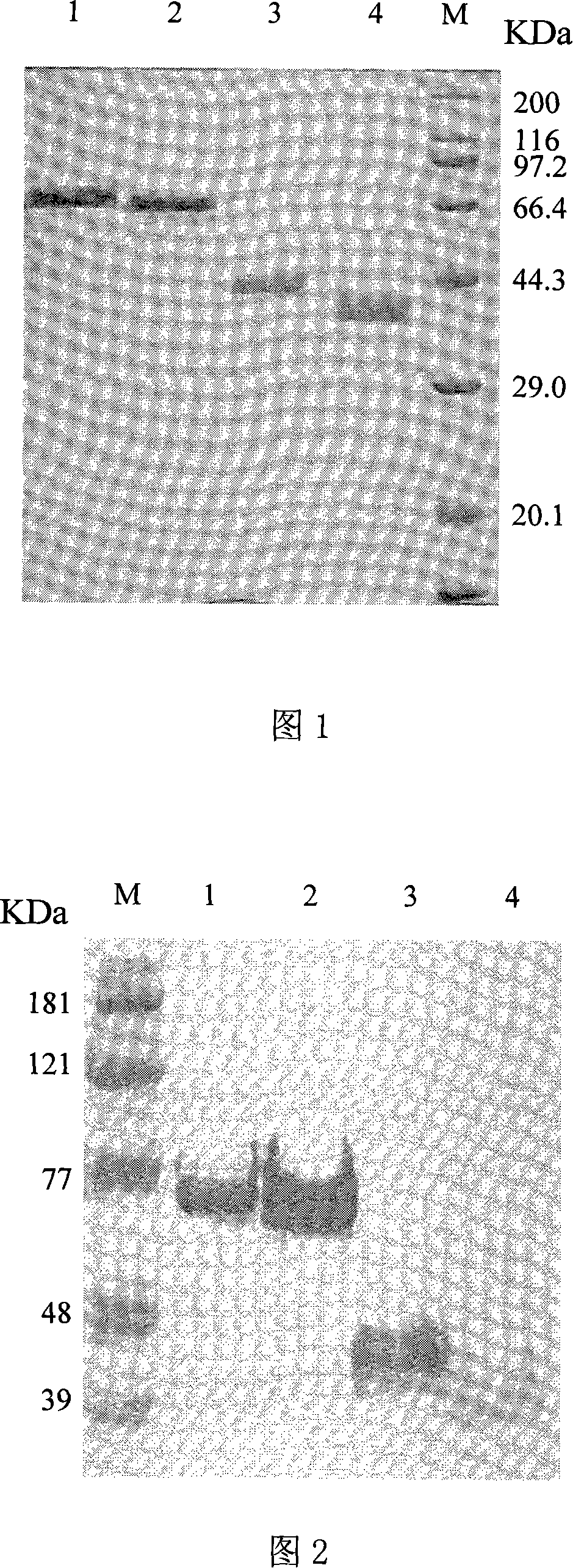
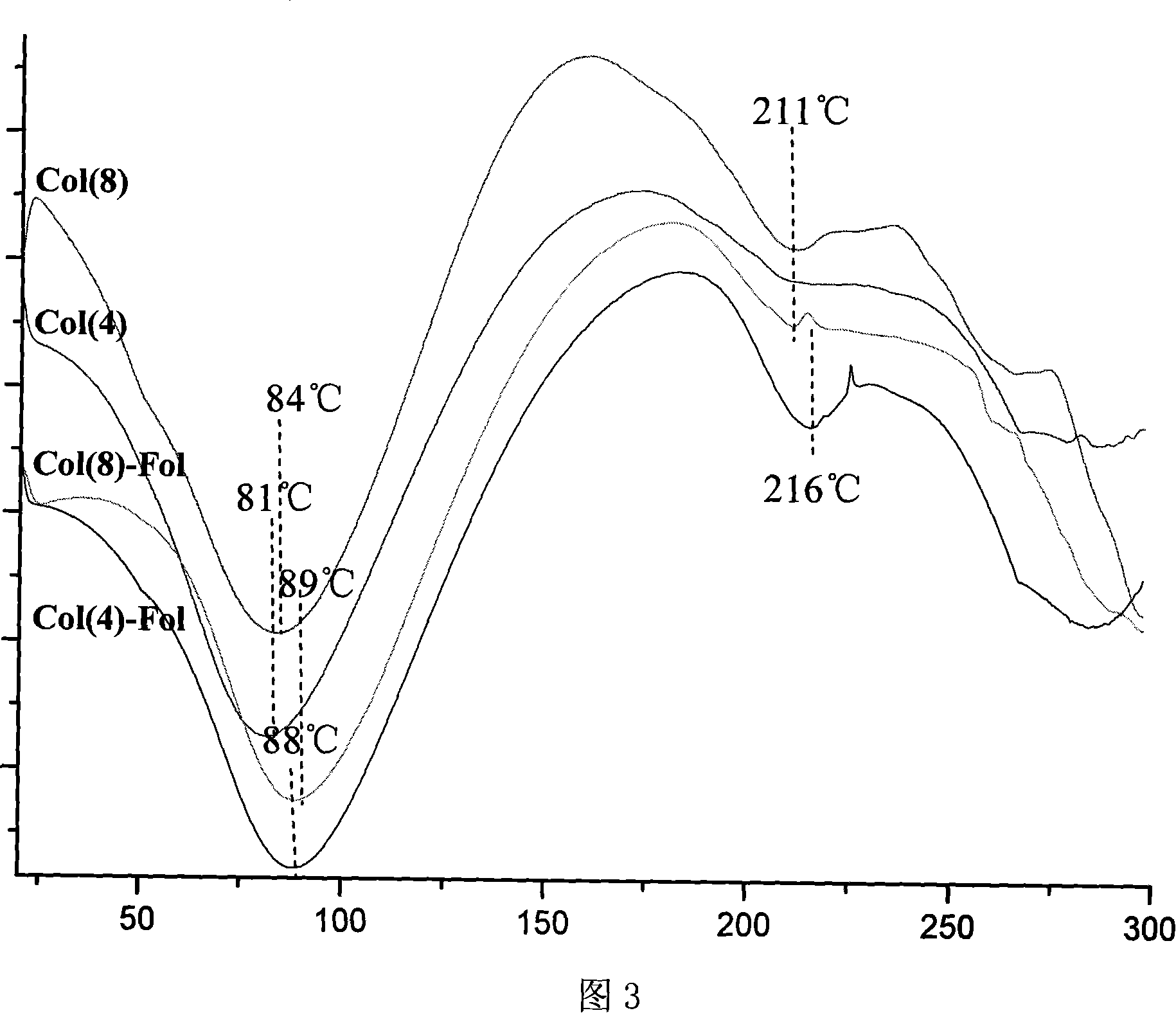
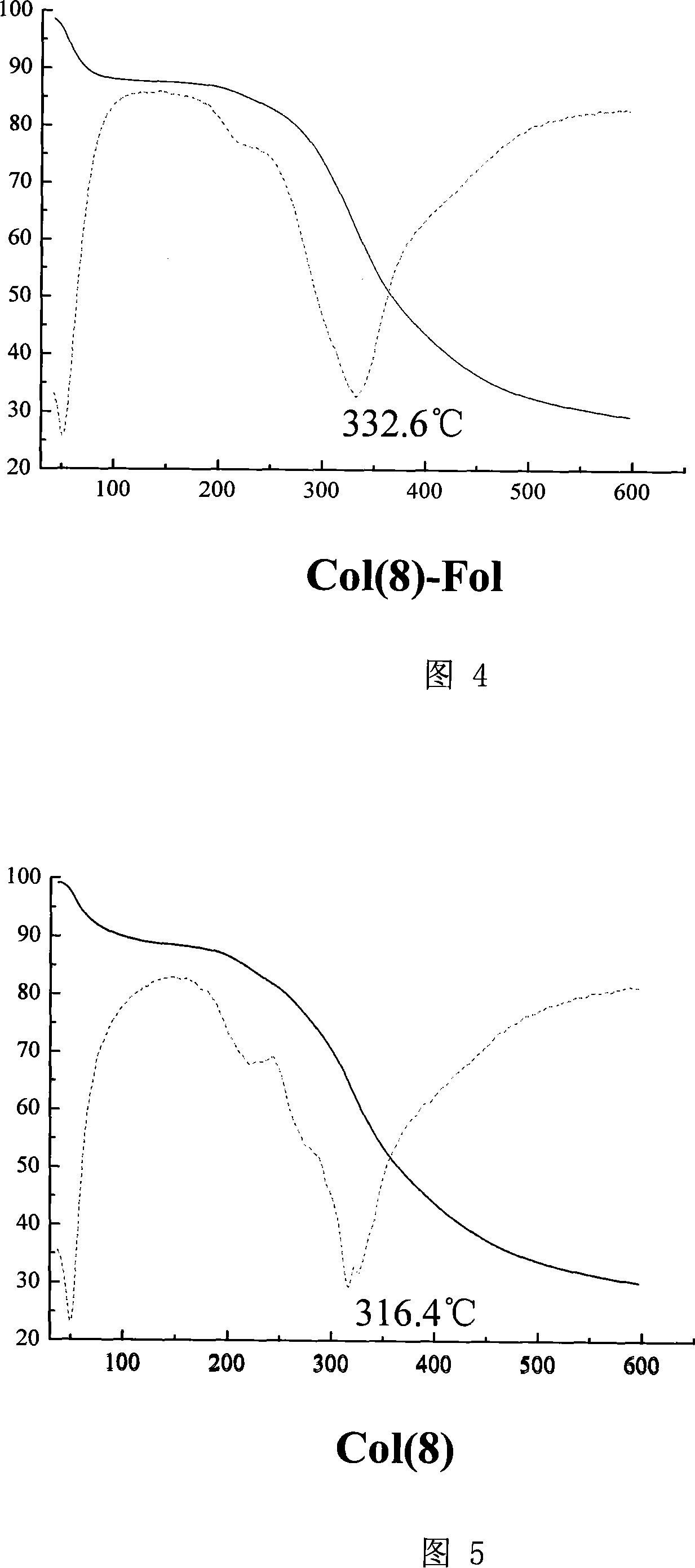
[0108] (3) Take 1-5 mg of recombinant human-like collagen for differential scanning calorimetry (DSC) (Figure 3), thermogravimetric (TGA) and micro-thermogravimetric (DTG) analysis (Figure 4-7).
[0109] Fig. 3 is the differential scanning calorimetry (DSC) spectrogram of the recombinant human-like collagen with different molecular weights of the embodiment of the present invention and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Heat denaturation temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com