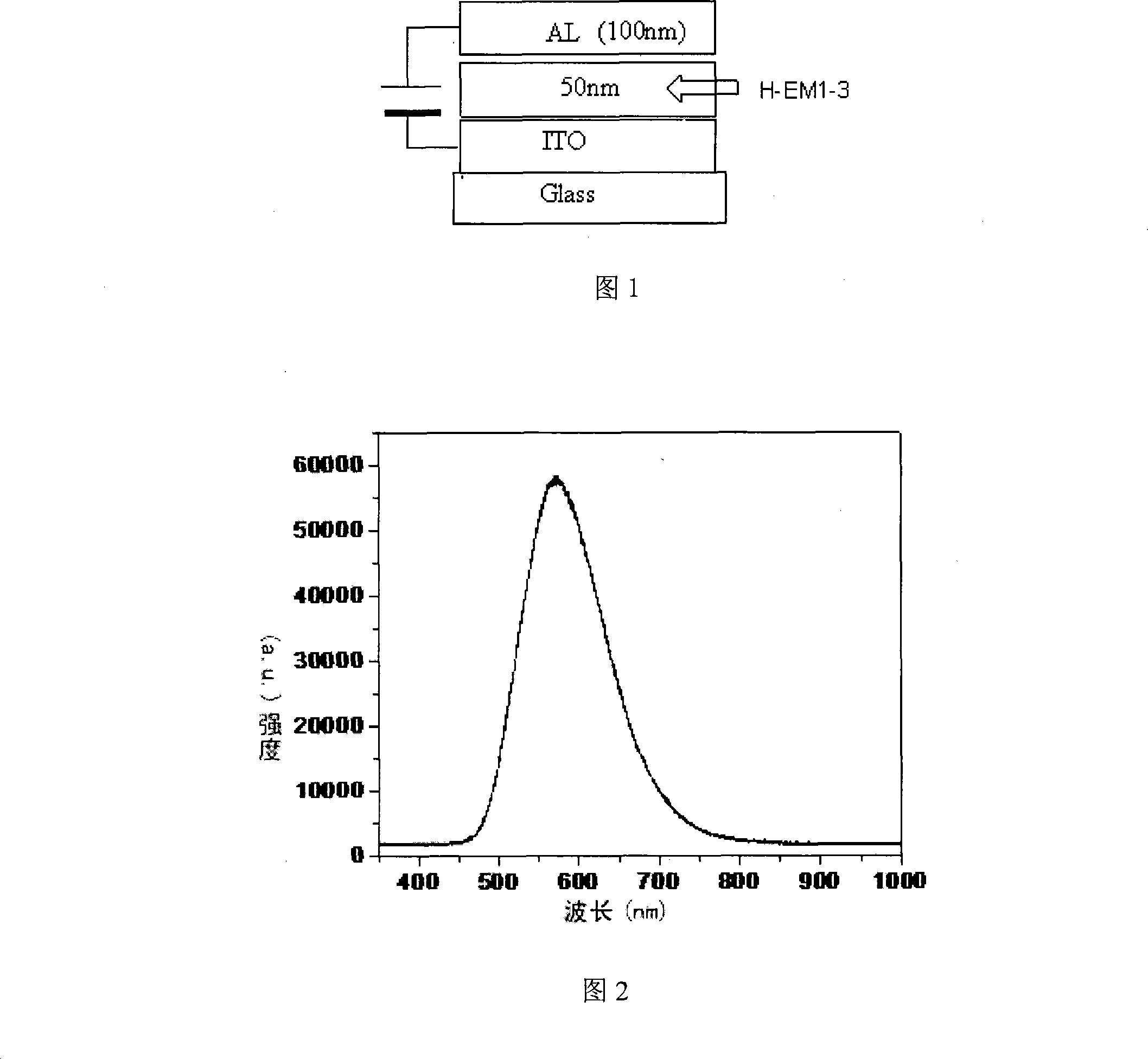
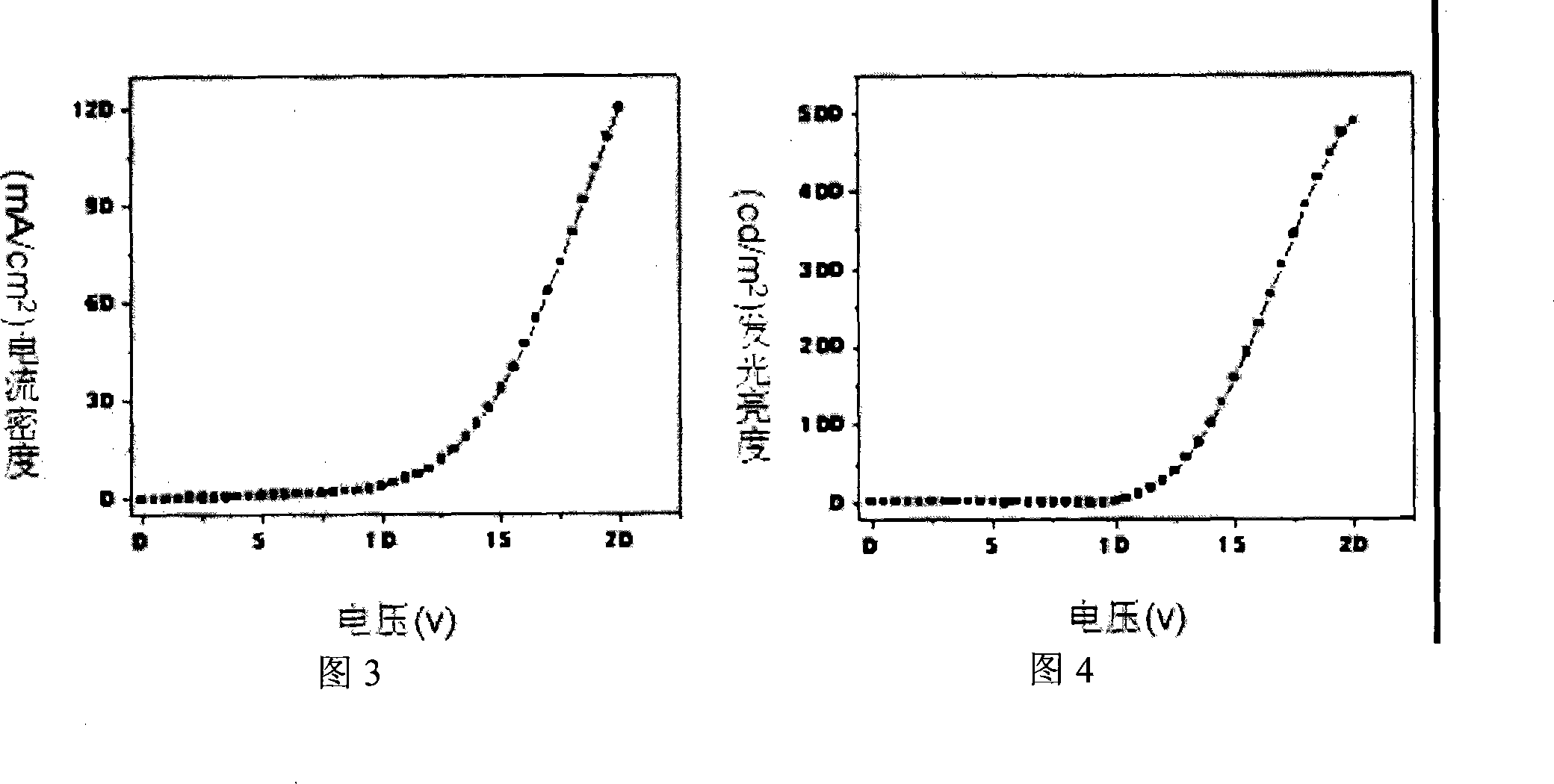
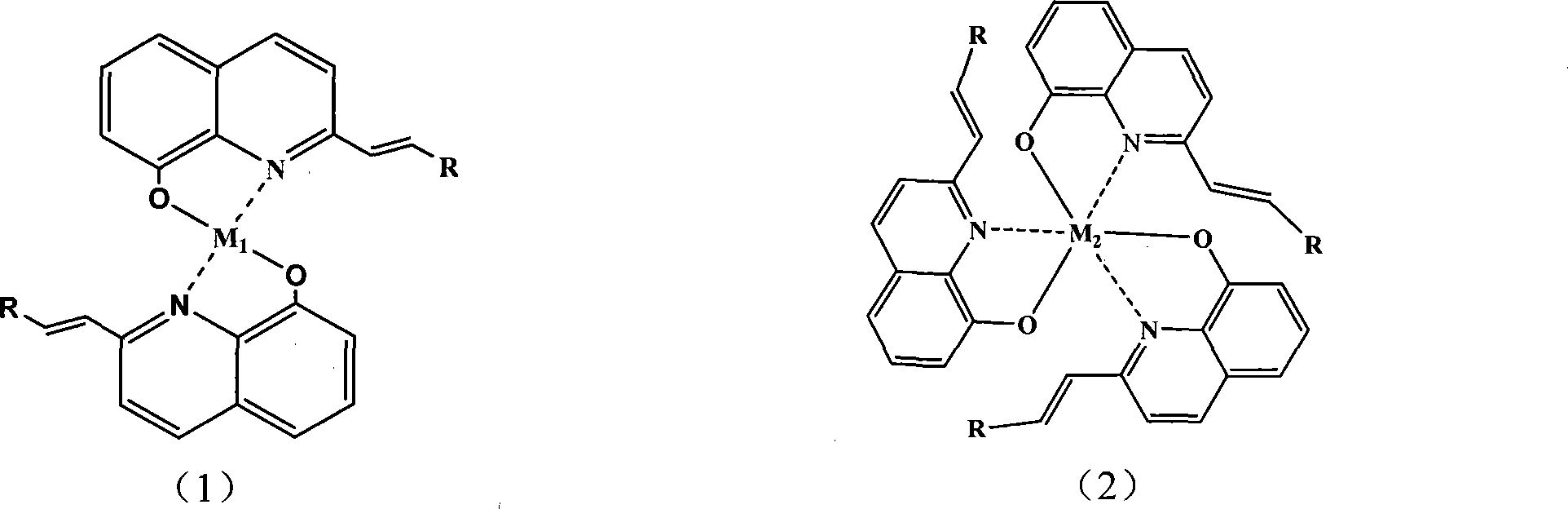
Single-layer organic luminescent device material capable of lightening yellow light and preparation method thereof
An organic light-emitting device, a single-layer technology, applied in the direction of light-emitting materials, chemical instruments and methods, electroluminescent light sources, etc., can solve the problems of reduced yield, expensive time-consuming and unfavorable assembly of devices, and achieve low cost and high efficiency. Yield, the effect of low requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of 2-[5'-(bromothiophene)-vinyl]-8-hydroxyquinoline (abbreviated as Ar-Q-1)
[0036] Weigh the 2-methyl-8-hydroxyquinoline 1 of 0.764g (4mmol), the 5-bromothiophene-2-aldehyde of 0.645g 4mmol g (4mmol), put into the there-necked flask, then add to the there-necked flask Add 15mL of acetic anhydride to make it completely dissolved in the three-necked flask, under the protection of nitrogen, heat at 134-138°C under stirring to make it reflux for about 40h. During the reaction, use thin-layer chromatography to track the reaction. After the reactant is cooled, put It was poured into 100 ml of ice water and stirred overnight. A yellow precipitate was obtained by centrifugation. Using ethyl acetate / petroleum ether as eluent (50:1 v / v) for column separation, 0.39 g of yellow needle-like substance Ar-Q-1 was obtained. Yield: 0.39 g, yield: 30%. Detection instrument and method are as follows (the detection instrument and method of following embodiment are...
Embodiment 2
[0037] Example 2: Bis-2-[5'-(bromothiophene)-vinyl]-8-hydroxyquinoline zinc (abbreviated as H-EM 1 -1) Synthesis
[0038] Weigh 0.0329g of zinc acetate into a 100ml round bottom flask, slowly add 20ml of 0.015mol / L anhydrous methanol solution of compound (Ar-Q-1) dropwise, and stir magnetically for 24h at room temperature to obtain a yellow turbid solution. Centrifuged, washed several times with methanol, vacuum-dried yellow solid H-EM-1; 0.098g, yield 90%. IR (KBr, cm -1 ): 3043.50 (v Ar-H ), 1618, 1451 (v C=C , v C=N ), 968 (trans-γ C-H out-of-plane bending vibration absorption); FAB MS: m / z: 727.
Embodiment 3
[0039] Example 3: Synthesis of 2-[5'-(bromodithiophene)-vinyl]-8-hydroxyquinoline (abbreviated as Ar-Q-2)
[0040] Weigh 0.764g (4mmol) of 2-methyl-8-hydroxyquinoline 1, 0.68g (4mmol) of 5-(5-bromothiophene)-2-thiophene-2-aldehyde, put it into a three-necked flask, Then add 15mL of acetic anhydride to the three-necked flask to completely dissolve it in the three-necked flask. Under nitrogen protection, heat at 134-138°C under stirring to make it reflux for about 40 hours. During the reaction, use thin-layer chromatography to track the reaction. After the reactant was cooled, it was poured into 100 ml of ice water and stirred overnight. A yellow precipitate was obtained by centrifugation. Using ethyl acetate / petroleum ether as eluent (25:1 v / v) for column separation, 0.168 g of yellow substance Ar-Q-2 was obtained. Yield: 0.168 g, Yield: 10% Rf = 0.76 (ethyl acetate / n-Hexane = 2:5). IR (KBr, cm -1 )3342.28 (v O-H );3049(v Ar-H ), 1653-1465 (v C=C , v C=N ); 1 HNMRδ: 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com