Homatropine hydrobromide in-situ gel rubber preparations for dripping eyes and preparation method thereof
The technology of homatropine hydrobromide and in-situ gel is applied in the directions of pharmaceutical formulation, drug delivery, sensory diseases, etc., and can solve the problems of large fluctuation of blood drug concentration, easy occurrence of adverse reactions, frequent administration times, etc. To achieve the effect of reducing the number of eye drops, reducing the loss of drugs, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
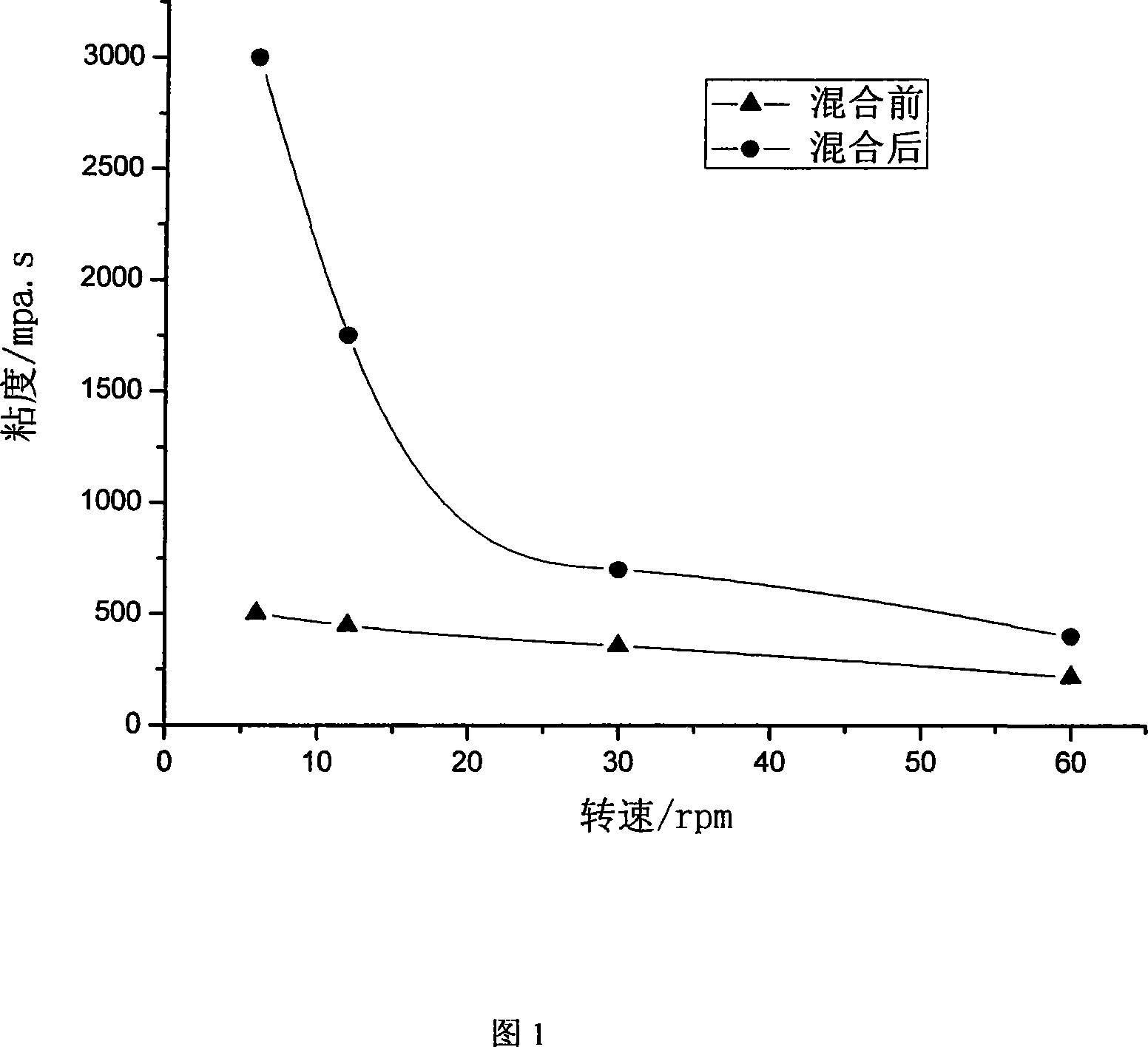
Image
Examples
Embodiment 1
[0029] Component Content (by mass)
[0030] Homatropine Hydrobromide 1%
[0031] Gellan Gum 0.5%
[0032] Benzalkonium Chloride 0.01%
[0033] 10% sodium hydroxide solution to adjust the pH value to 6.5-7.2
[0034] Mannitol 3.5%
[0035] Ultrapure water balance
[0036] Take 80g of deionized water, add 0.5g of gellan gum dispersedly while stirring, and stir at about 90°C for about 15 minutes to completely dissolve the gellan gum to form a transparent solution, cool to room temperature, then add 1g of hydrobromic acid and immediately Tropin, 0.01g benzalkonium chloride, and 3.5g mannitol were stirred and dissolved, and the pH was adjusted to 6.5-7.2 by adding 10% sodium hydroxide solution, and ultrapure water was added to 100g.
Embodiment 2
[0038] Component Content (by mass)
[0039] Homatropine Hydrobromide 1%
[0040] Gellan Gum 0.6%
[0041] Benzalkonium Bromide 0.01%
[0042] 10% sodium hydroxide solution to adjust the pH value to 6.5-7.2
[0043] Sucrose 6%
[0044] Ultrapure water balance
[0045] Take 60g of deionized water, add 0.6g of gellan gum dispersedly while stirring, and stir at about 90°C for about 15 minutes to completely dissolve the gellan gum to form a transparent solution, cool to room temperature, then add 1g of hydrobromic acid, and immediately Tropin, 0.01g benzalkonium bromide, 6g sucrose, stirred and dissolved, adjusted pH to 6.5-7.2 by adding 10% sodium hydroxide solution, supplemented with ultrapure water to 100g.
Embodiment 3
[0047] Component Content (by mass)
[0048] Homatropine Hydrobromide 2%
[0049] Gellan Gum 0.4%
[0050] Methylparaben 0.02%
[0051] Propylparaben 0.01%
[0052] 10% sodium hydroxide solution to adjust the pH value to 6.5-7.2
[0053] Glycerol 2%
[0054] Ultrapure water balance
[0055] Take 80g of deionized water, add 0.4g of gellan gum dispersedly while stirring, and stir at about 90°C for about 15min to completely dissolve the gellan gum to form a transparent solution, cool to room temperature, then add 2g of hydrobromic acid and immediately Tropin, 0.02g methylparaben, 0.01g propylparaben, 2g glycerol, after stirring and dissolving, adjust the pH to 6.5-7.2 by adding 10% sodium hydroxide solution, and add ultra-pure water to 100g .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com