Spaston orally disintegrating tablets and preparation thereof
The technology of oral disintegrating tablet and phloroglucinol is applied in directions such as pharmaceutical formulations, medical preparations of inactive ingredients, and pill delivery, which can solve problems such as poor dissolution effect, achieve fast onset of effect, fast absorption, and reduce local stimulating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
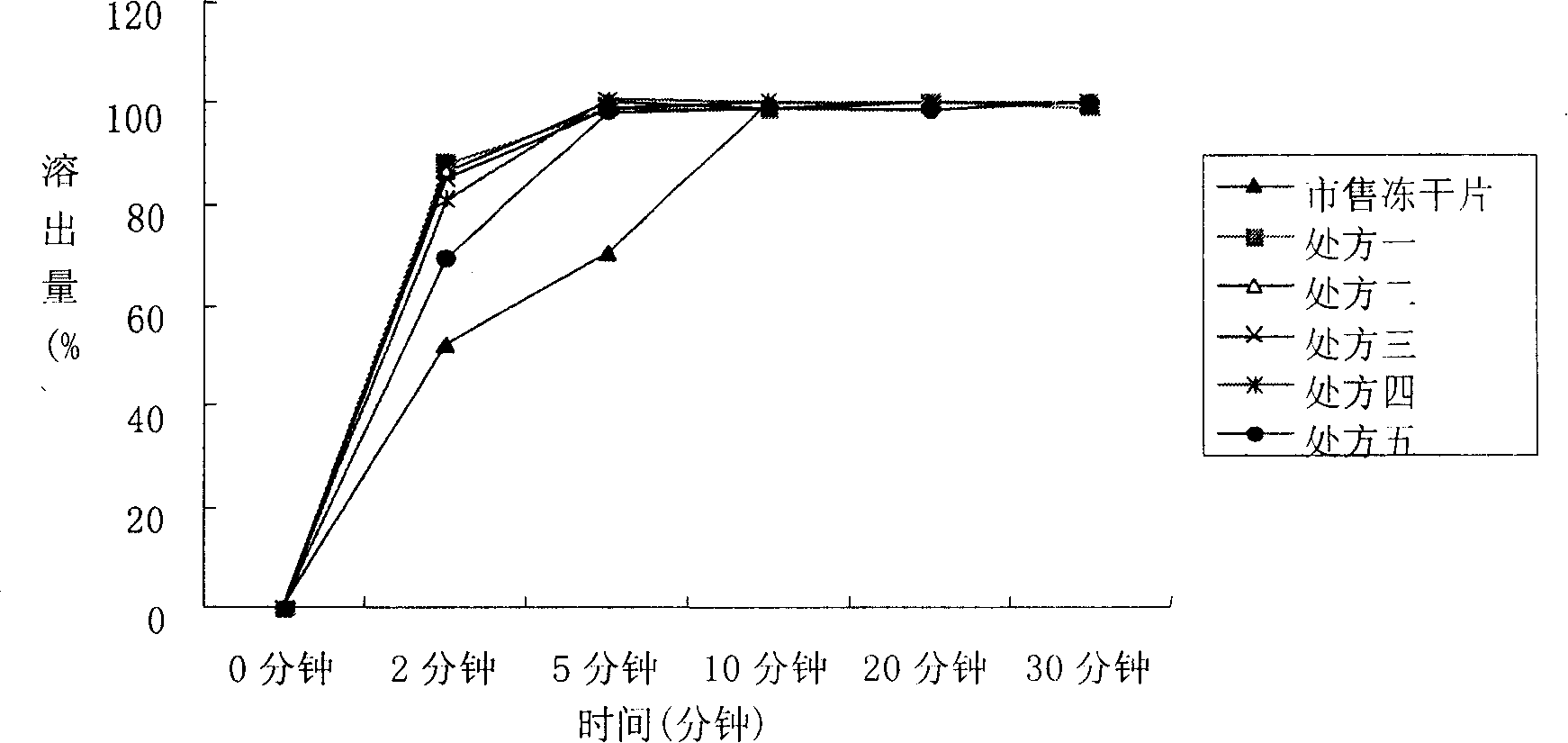
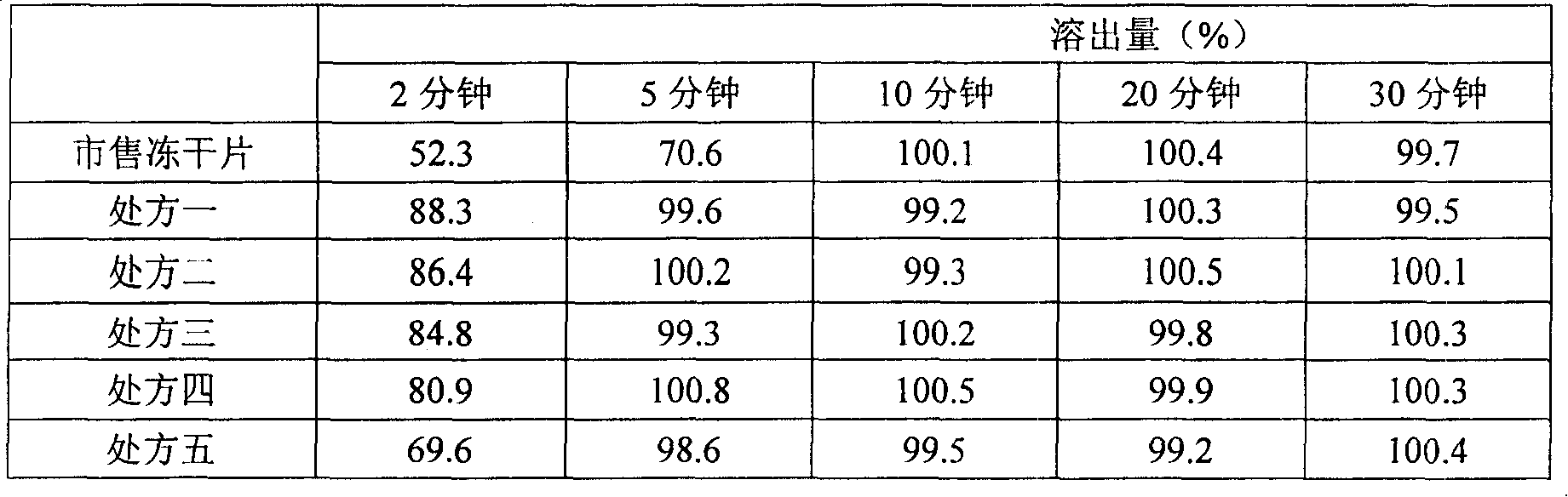
Image
Examples
Embodiment 1
[0034] Embodiment 1 Preparation of orally disintegrating tablet of the present invention
[0035] 1. Prescription
[0036] 1. Raw material - Phloroglucinol dihydrate 80.0g
[0037] 2. Filling agent - mannitol 20.0g
[0038] 3. Disintegrant—cross-linked polyvinylpyrrolidone (PVPP) 30.0g
[0039] Microcrystalline cellulose 20.0g
[0040] 4. Lubricant—magnesium stearate 0.75g
[0041] A total of 1000 tablets were prepared, specification: 80.0mg (calculated as phloroglucinol dihydrate)
[0042] 2. Preparation method
[0043] Step 1: Pass phloroglucinol dihydrate and auxiliary materials through a 100-mesh sieve respectively, and take by weight.
[0044] The second step: get the cross-linked polyvinylpyrrolidone (PVPP) of phloroglucinol dihydrate, mannitol, microcrystalline cellulose and 2 / 3 recipe quantity, mix well, add 50% ethanol (V / V) aqueous solution preparation Soft material, pass through 20 mesh sieve to granulate, dry at 50℃ for 4 hours, and granulate.
[0045] Step...
Embodiment 2
[0050] Example 2 Preparation of orally disintegrating tablet of the present invention
[0051] 1. Prescription
[0052] 1. Raw material - Phloroglucinol dihydrate 80.0g
[0053] 2. Filling agent - mannitol 14.0g
[0054] 3. Disintegrating agent - cross-linked polyvinylpyrrolidone (PVPP) 25.0g
[0055]Microcrystalline cellulose 14.0g
[0056] 4. Lubricant - Micropowder silica gel 2.0g
[0058] 5. Flavoring agent - sucralose 1.0g
[0059] A total of 1000 tablets were prepared, specification: 80.0mg (calculated as phloroglucinol dihydrate)
[0060] 2. Preparation method
[0061] Step 1: Pass phloroglucinol dihydrate and auxiliary materials through a 100-mesh sieve respectively, and take by weight.
[0062] The second step: get phloroglucinol dihydrate, mannitol, sucralose and 60% prescription amount of cross-linked polyvinylpyrrolidone (PVPP) and 60% prescription amount of microcrystalline cellulose, mix well, add 50% Soft materials made from et...
Embodiment 3
[0068] Embodiment 3 Preparation of orally disintegrating tablet of the present invention
[0069] 1. Prescription
[0070] 1. Raw material - Phloroglucinol dihydrate 80.0g
[0071] 2. Filling agent - mannitol 20.0g
[0072] 3. Disintegrating agent - cross-linked polyvinylpyrrolidone (PVPP) 20.0g
[0073] Microcrystalline cellulose 15.0g
[0074] 4. Lubricant - Micropowder silica gel 2.0g
[0075] Talc powder 2.0g
[0076] 5. Flavoring agent - sucralose 1.0g
[0077] A total of 1000 tablets were prepared, specification: 80.0mg (calculated as phloroglucinol dihydrate)
[0078] 2. Preparation method
[0079] Step 1: Pass phloroglucinol dihydrate and auxiliary materials through a 100-mesh sieve respectively, and take by weight.
[0080] The second step: get phloroglucinol dihydrate, mannitol, sucralose and 60% prescription amount of cross-linked polyvinylpyrrolidone (PVPP) and 60% prescription amount of microcrystalline cellulose, mix well, add 50% Soft materials made fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com