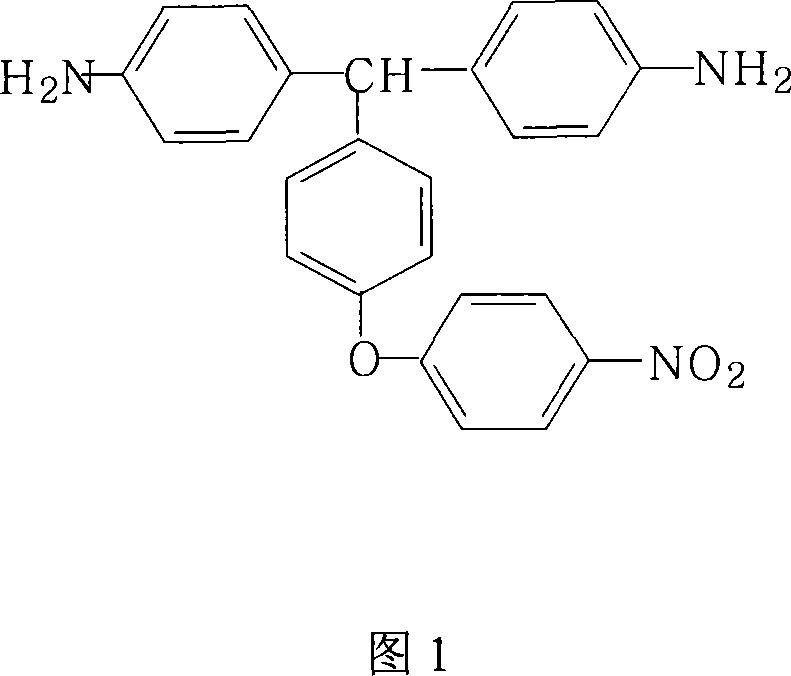
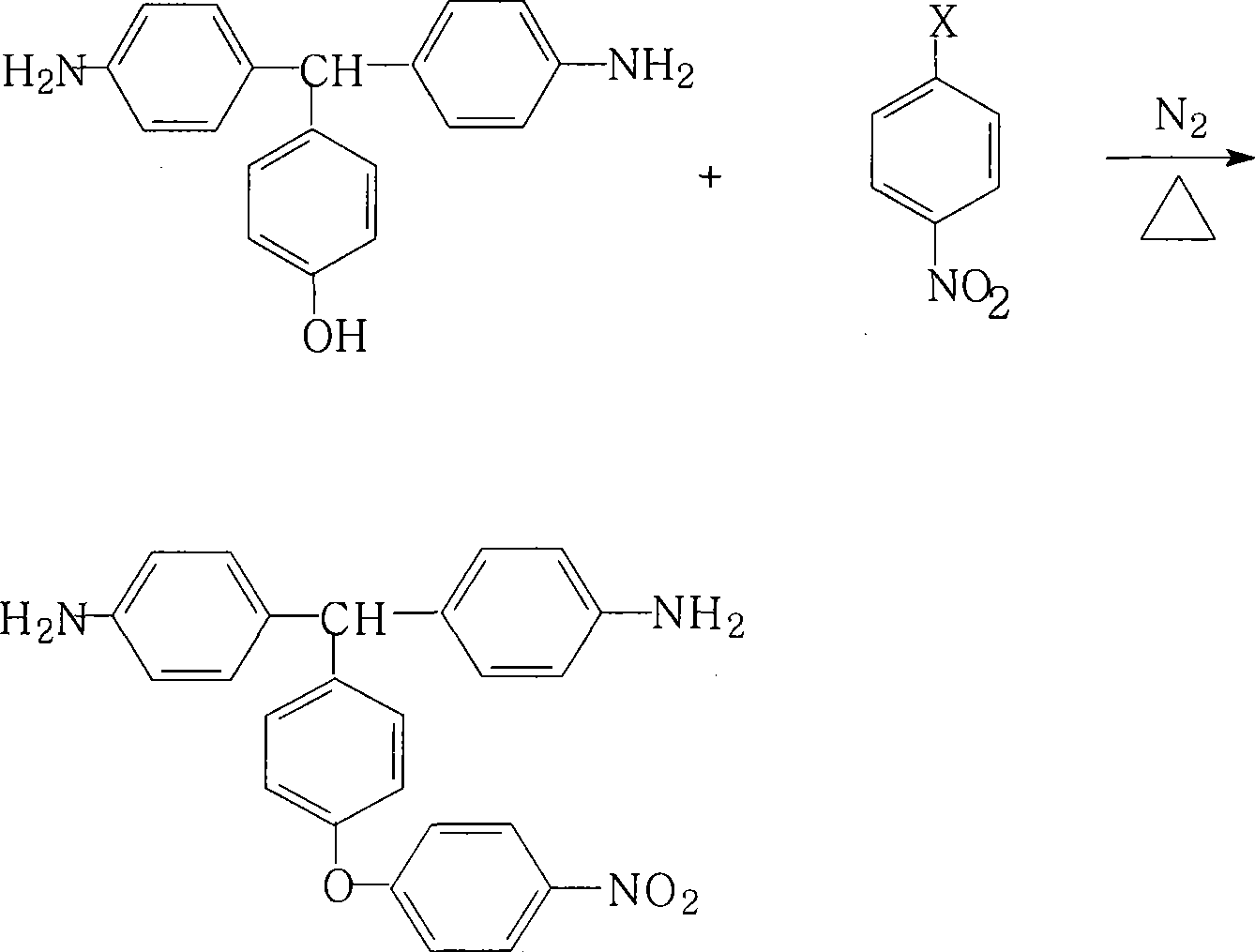
Preparation method for 4,4'-diamino-4''-(4-nitrophenoxy)triphenylmethane
A technology of nitrophenoxy and hydroxytriphenylmethane, which is applied in 4 fields, can solve problems such as no published literature or patent reports, and achieve the effects of easy recycling, less three wastes, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 29.0 grams (0.1 moles) of 4,4'-diamino-4"-hydroxytriphenylmethane, 18.9 grams (0.12 moles) of 4-chloronitrobenzene (4CNB), 24.8 grams (0.18 moles) of potassium carbonate, 252 ml N,N-dimethylformamide (DMF) and 84 milliliters of toluene were added in the round-bottomed flask with thermometer, nitrogen pipe, oil-water separator, reflux condenser and mechanical stirrer, and after nitrogen was stirred at room temperature for 0.5 hour, After heating to 100°C for 3 hours, gradually increase the reaction temperature, and finally rise to 150°C for 1 hour, and maintain the reaction at 150°C for 16 hours, then cool to 80°C, filter while it is hot, remove the filter residue, and reduce the mother liquor Concentrate under reduced pressure, cool, crystallize, filter, and dry to obtain 4,4'-diamino-4"-(4-nitrophenoxy)triphenylmethane, according to 4,4'-diamino-4"-hydroxy The feeding amount and product amount of triphenylmethane were determined, and the yield was 91% (purity 98.1%). ...
Embodiment 2
[0027] Add 29.0 g (0.1 mol) of 4,4′-diamino-4″-hydroxytriphenylmethane, 0.12 mol of potassium hydroxide, 180 ml of N-methylpyrrolidone (NMP) and 60 ml of xylene to a thermometer, nitrogen Tube, oil-water separator, reflux condenser and mechanical stirrer round-bottomed flask, after 0.5 hours of nitrogen gas stirring at room temperature, heating and reflux until no water is analyzed, cooling the reaction system to 90 ° C, adding 14.1 g (0.10 mol) 4-fluoronitrobenzene (4FNB), heating and stirring, after reacting at 120°C for 3 hours, gradually increase the reaction temperature, and finally rise to 150°C for 1 hour, and maintain the reaction at 150°C for 10 hours, then cool to 80°C, filter while hot, remove the filter residue, concentrate the mother liquor under reduced pressure, cool, crystallize, filter, and dry to obtain 4,4'-diamino-4"-(4-nitrophenoxy)triphenylmethane, according to 4,4'-diamino-4"-hydroxytriphenylmethane was measured for the amount of feed and product, and th...
Embodiment 3
[0029] 29.0 g (0.1 mol) of 4,4′-diamino-4″-hydroxytriphenylmethane, 0.12 mol of 4-bromonitrobenzene (4BNB), 0.08 mol of potassium carbonate, 120 ml of N,N-dimethylformaldehyde Amide (DMF) and 60 ml of toluene were added to a round-bottomed flask equipped with a thermometer, nitrogen tube, oil-water separator, reflux condenser and mechanical stirrer. After stirring with nitrogen at room temperature for 0.5 hours, the temperature was raised to 120 ° C for 3 hours. After that, gradually increase the reaction temperature, and finally rise to 150°C for 1 hour, and maintain the reaction at 150°C for 12 hours, then cool to 80°C, filter while it is hot, remove the filter residue, concentrate the mother liquor under reduced pressure, cool, crystallize, and filter , oven dry, obtain 4,4'-diamino-4 "-(4-nitrophenoxy) triphenylmethane, according to the charging amount and product of 4,4'-diamino-4 "-hydroxytriphenylmethane Quantitative determination, yield 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com