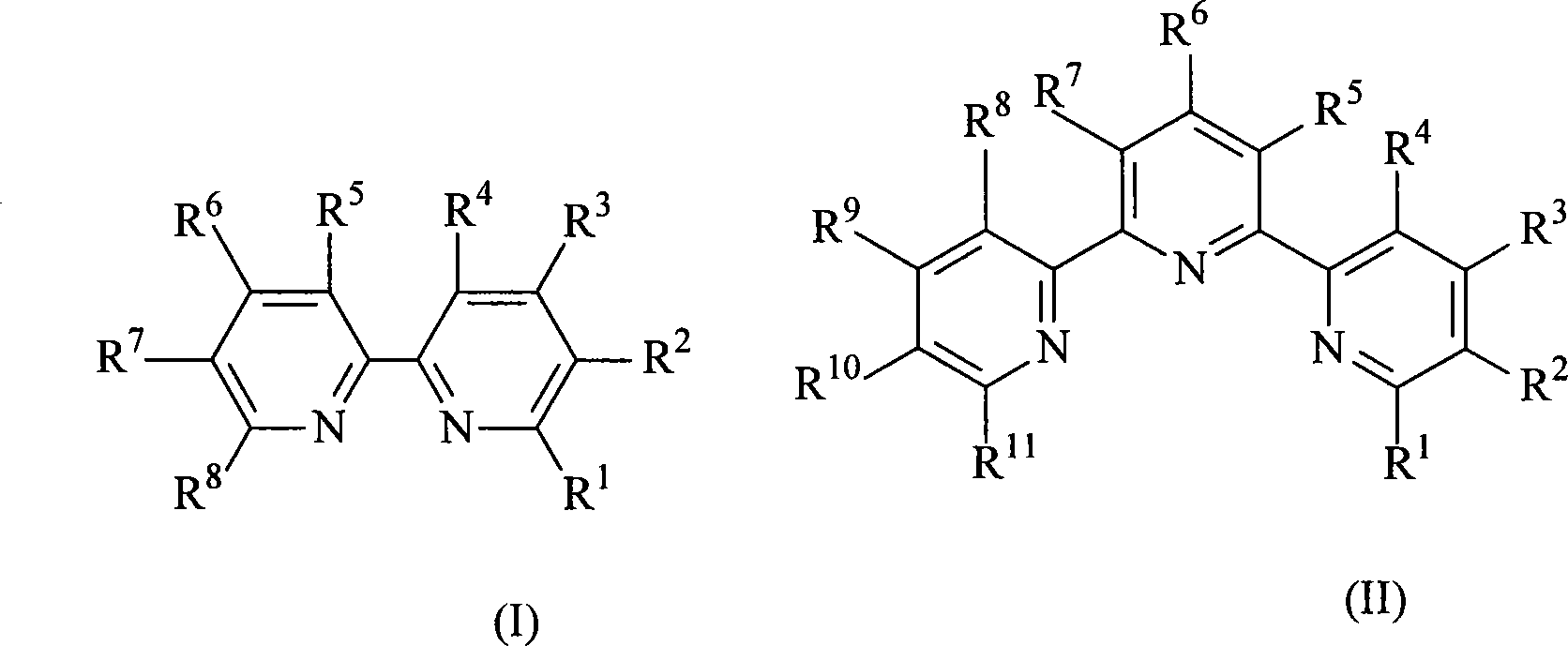
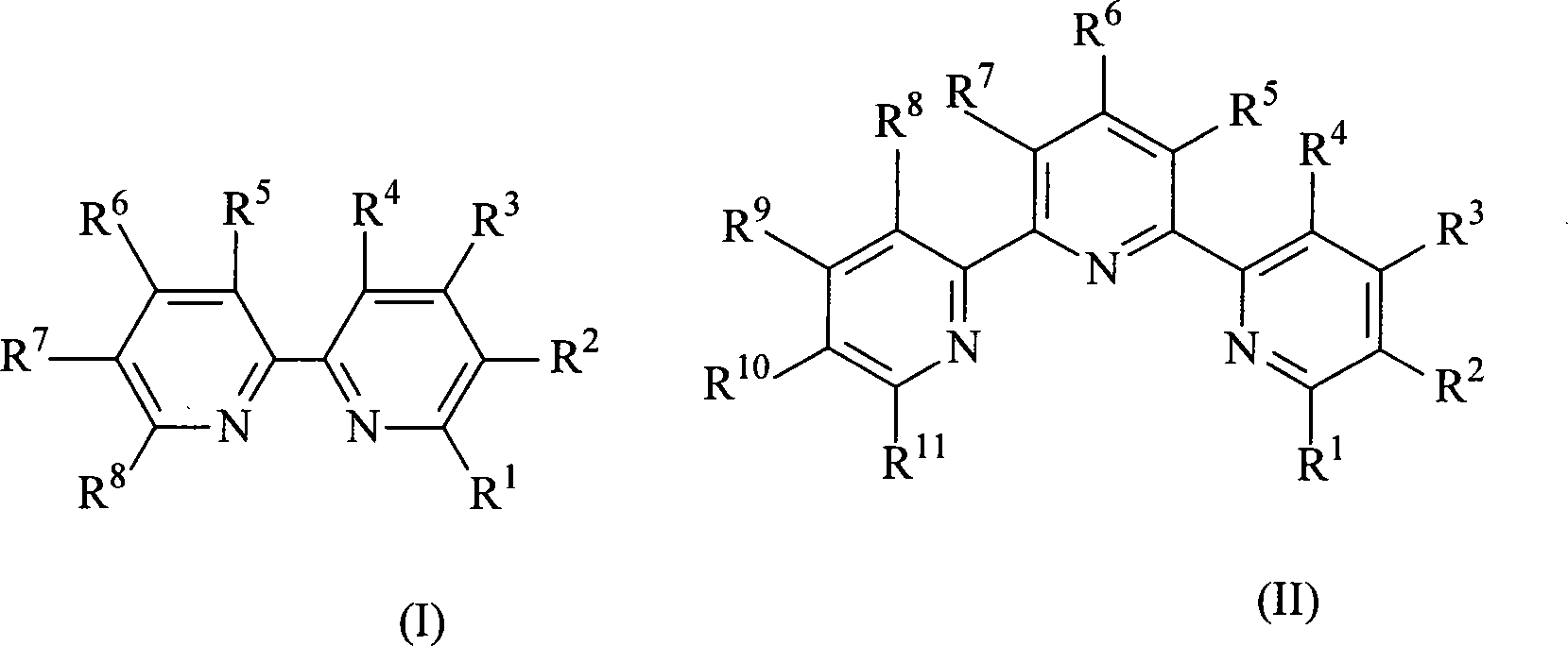
Ruthenium polypyridyl complexes and synthesis method for derivatives thereof
A kind of synthetic method, the technique of ruthenium polypyridine, applied in the field of synthetic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Ru(dcbpy) 2 Cl 2 The synthesis, wherein dcbpy=2,2'-bipyridine-4,4'-dicarboxylic acid will 420mg of RuCl 3 ·3H 2 O and 791 mg of polypyridine ligand dcbpy (ie 2,2'-bipyridine-4,4'-dicarboxylic acid) were mixed in 150 ml of DMF (ie N,N-dimethylformamide), and loaded into a polytetrafluoroethylene In a stainless steel reactor lined with vinyl fluoride, high-purity N 2 Gas was bubbled for 15 minutes, dissolved oxygen was removed, the reactor was sealed, and heated to 190°C for 8 hours. After cooling, the resulting mixture was filtered, the filtrate was rotary evaporated to remove the DMF solvent, the obtained solid was recrystallized with acetone, the obtained crystal was filtered and vacuum-dried at 50°C for 3 hours to obtain the product Ru(dcbpy) 2 Cl 2 . Yield 85%.
[0048] Elemental analysis calculated value (C 24 h 16 N 4 o 8 Cl 2 Ru): C, 43.67; H, 2.44; N, 8.48; Cl, 10.74 (%). Measured values: C, 44.03; H, 2.50; N, 8.91; Cl, 10.70 (%).
Embodiment 2
[0050] Synthesis of cis-diisothiocyanato-bis(2,2'-bipyridyl-4,4'-dicarboxylic acid)ruthenium
[0051] Will Ru(dcbpy) 2 Cl 2 2H 2 O 850 mg was dissolved in 90 ml of DMF (protected from light), 60 ml of 0.1M NaOH aqueous solution was added thereto, 5.5 g of KSCN was dissolved in 10 ml of water and added to the above solution, and the resulting solution was loaded into a stainless steel tank with a Teflon lining In the reactor, with high-purity N 2 Gas was bubbled for 15 minutes to remove dissolved oxygen therein, the reactor was sealed, heated to 190° C., and kept for 6 hours under magnetic stirring conditions. After the reaction solution was cooled, the solvent was removed by rotary evaporation, and the resulting solid was dissolved in water and filtered through a sintered glass funnel, and the filtrate was washed with dilute HClO 4 Adjust the pH to 2.5, and after standing for 12 hours, suction filter to obtain a solid, and then use HClO with pH=3.5 4 Washed with aqueous s...
Embodiment 3
[0054] With embodiment 2 gained solid E, Ru(dcbpy) 2 (NCS) 2 Use LH-20 gel chromatographic column for chromatographic purification, using methanol as the eluent, the obtained dark purple band is recrystallized with ethanol to obtain Ru(dcbpy) 2 (NCS) 2 . Yield 70%.
[0055] Elemental analysis calculated value (C 26 h 16 N 6 S 2 o 8 Ru·2H 2 O): C, 42.1; H, 2.7; N, 11.3 (%). Measured values: C, 41.3; H, 2.7; N, 11.0 (%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com