Method for producing pitavastatin calcium raw material
A technology for pitavastatin calcium and raw materials, which is applied in the field of preparation of cholesterol-lowering drugs, can solve the problems of difficult separation of pitavastatin calcium raw materials, and achieves the effects of low raw material requirements, high product conversion rate and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
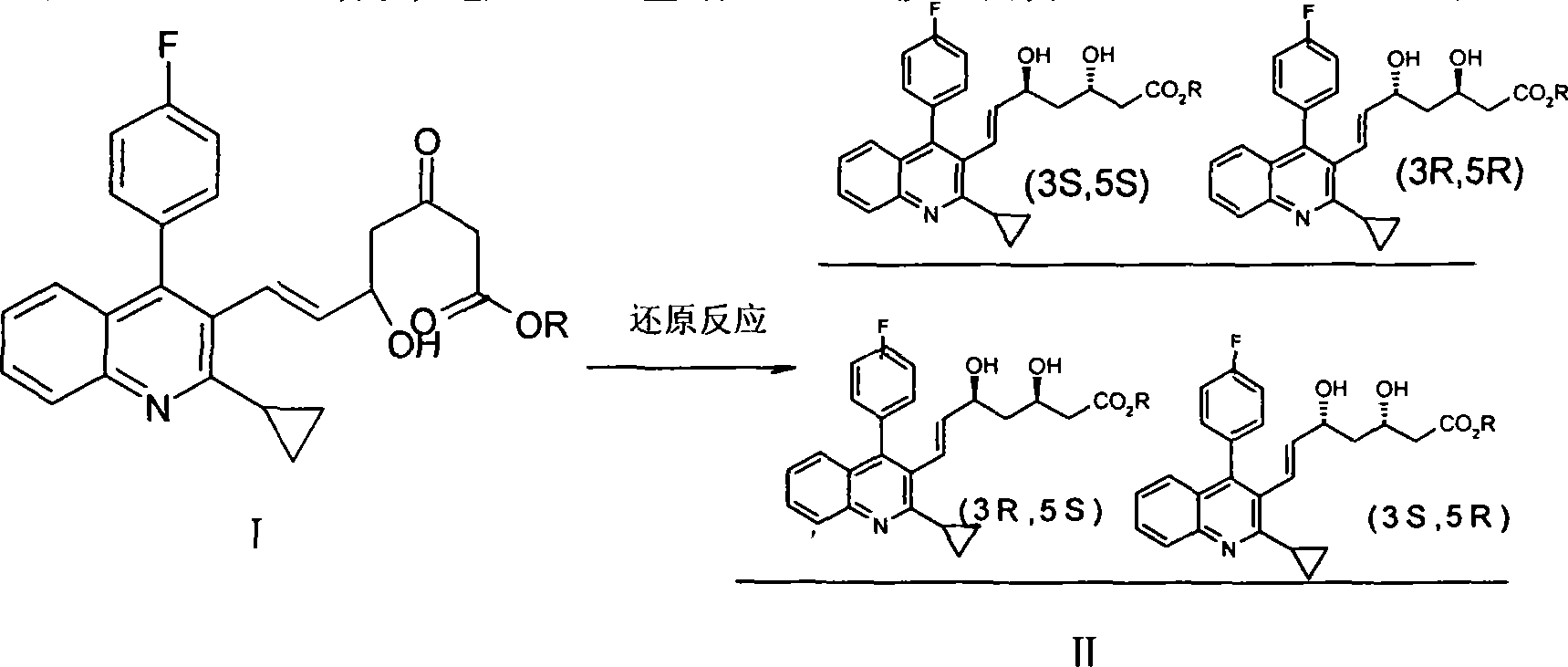
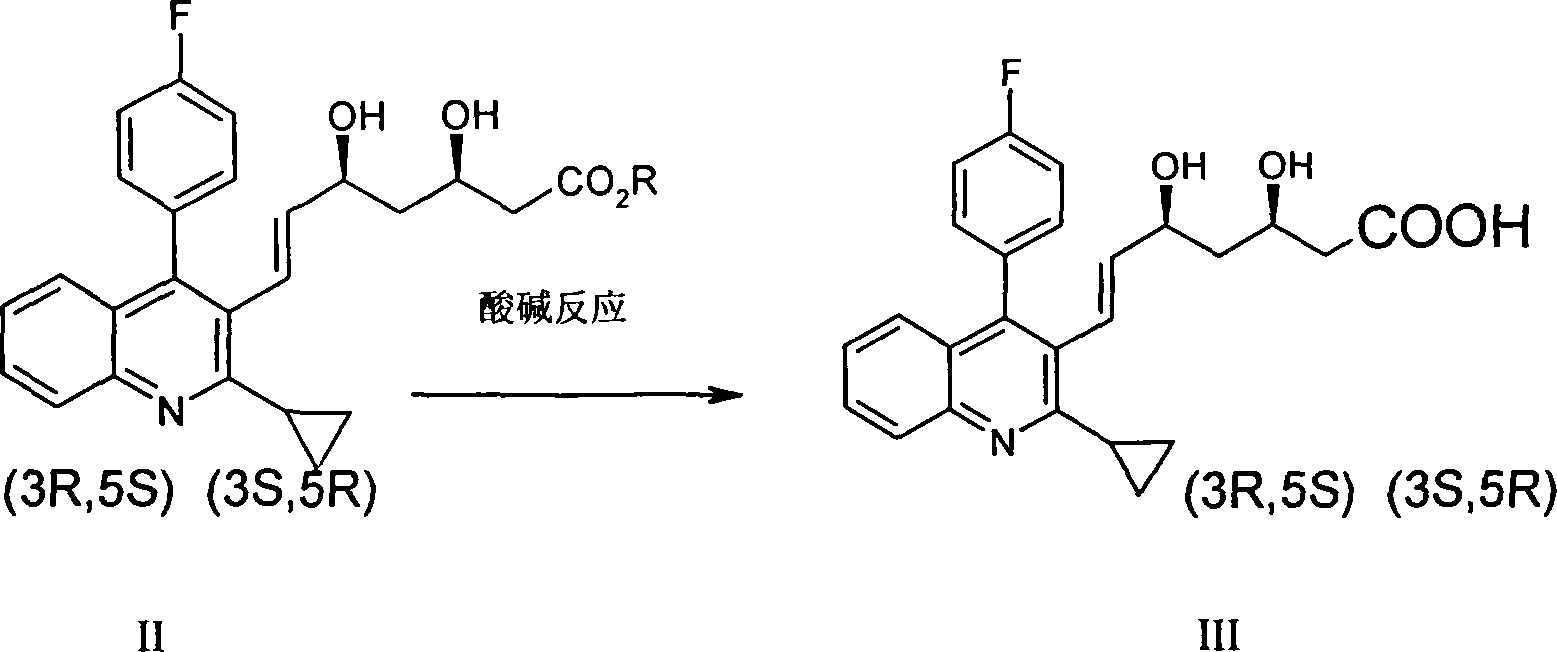
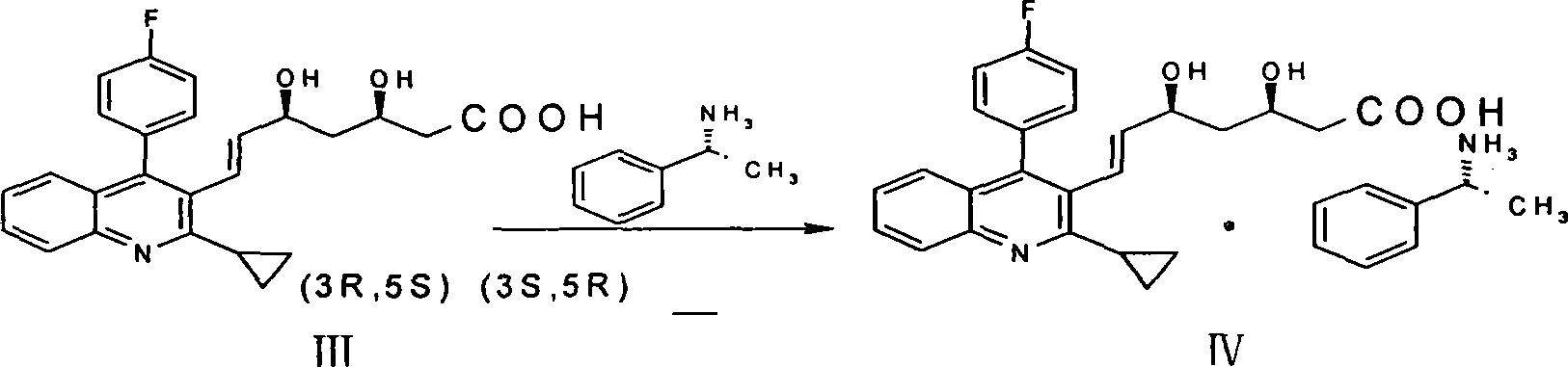
[0030] A method for preparing pitavastatin calcium raw material, with (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-5-hydroxyl-3-carbonyl- 6-heptenoic acid ethyl ester (I) is starting raw material, comprises the following steps:
[0031] The first step: (+)-3,5-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-heptenoic acid ethyl The preparation of ester, reaction formula:
[0032]
[0033] Operation process:
[0034] Add 770ml of anhydrous methanol, 5060ml of anhydrous tetrahydrofuran, and 253g of (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-5-hydroxyl to a 10L three-necked flask -3-carbonyl-6-heptenoic acid ethyl ester, under the protection of nitrogen, add dropwise 240ml of 50% diethylmethoxyborane / tetrahydrofuran solution, drop the temperature of the reaction solution to -50°C, and stir for 1 hour , add 42.8g of sodium borohydride, add dropwise 6150ml of ethyl acetate to the reaction liquid for extraction, wash with 5600ml of saturated salt ...
Embodiment 2
[0044] The first step: (+)-3,5-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-heptenoic acid ethyl Preparation of esters:
[0045] Operation process:
[0046] Add 770ml of anhydrous methanol, 5060ml of anhydrous tetrahydrofuran, and 253g of (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-5-hydroxyl to a 10L three-necked flask -3-carbonyl-6-heptenoic acid ethyl ester, under the protection of nitrogen, add 240ml of 50% trimethylborane / tetrahydrofuran solution dropwise, after dropping, cool the reaction solution to -100°C, stir for 1 hour, add 42.8 g potassium borohydride, add dropwise to the reaction solution, add 6150ml ethyl acetate to extract, wash with 5600ml saturated salt and 5600ml saturated aqueous sodium bicarbonate solution, concentrate under reduced pressure to remove solvent, add 4500ml methanol to dissolve, concentrate again, repeat the above steps three times , to obtain solid (+) (E)-3,5-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl...
Embodiment 3
[0054] The first step: (+)-3,5-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-heptenoic acid ethyl Preparation of esters:
[0055] Operation process:
[0056] Add 770ml of anhydrous methanol, 5060ml of anhydrous tetrahydrofuran, and 253g of (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-5-hydroxyl to a 10L three-necked flask -Ethyl 3-carbonyl-6-heptenoate, under the protection of nitrogen, add 240ml of 50% trimethoxyborane / tetrahydrofuran solution dropwise, after dropping, cool the reaction solution to -50°C, stir for 1 hour, and add 42.8g of boron Add potassium hydride dropwise to the reaction solution, add 6150ml of ethyl acetate for extraction, wash with 5600ml of saturated salt and 5600ml of saturated aqueous sodium bicarbonate solution. Concentrate under reduced pressure to remove solvent, add 4500ml of methanol to dissolve, concentrate again, repeat the above steps three times to get Solid (+) (E)-3,5-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com