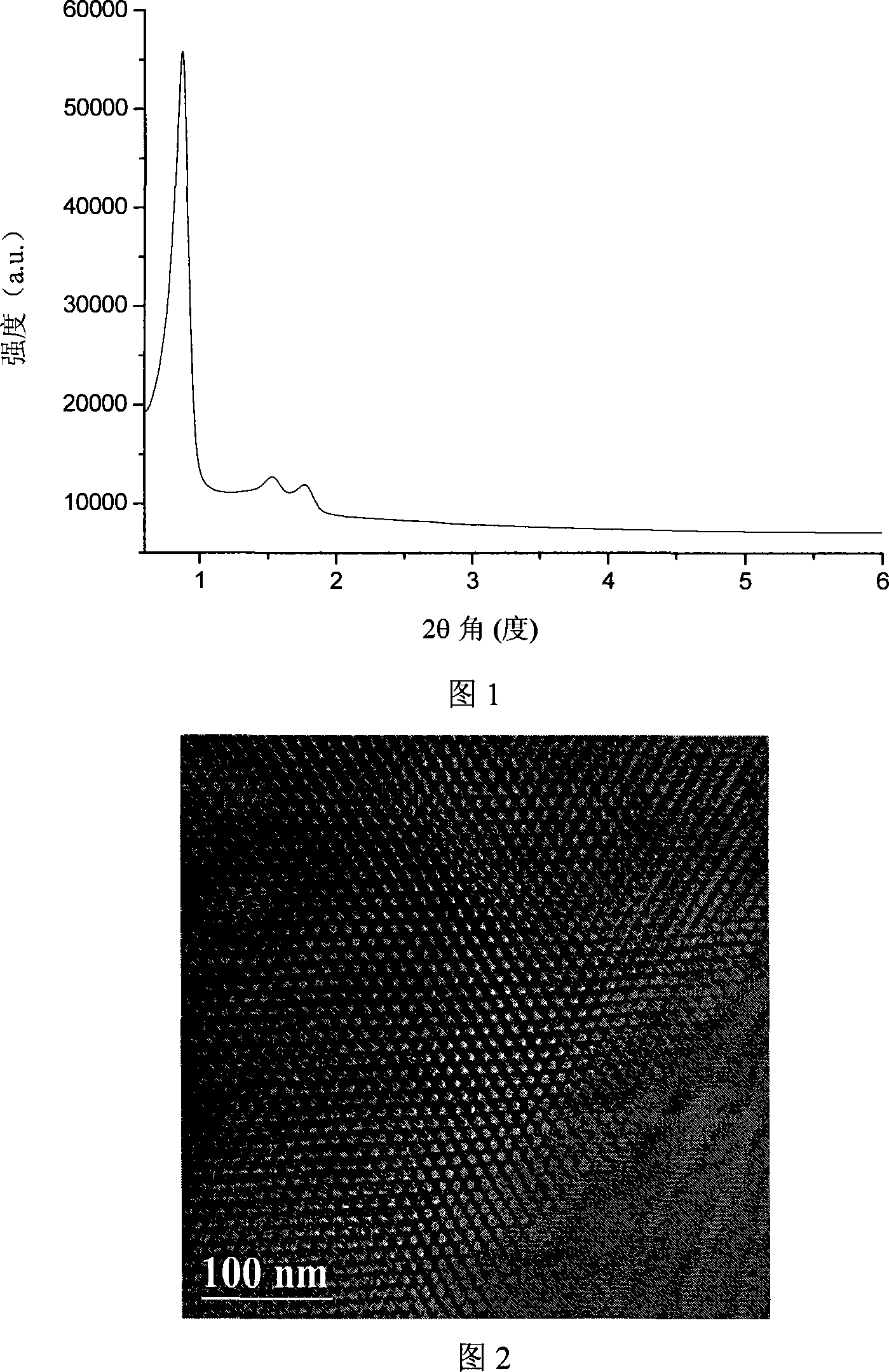
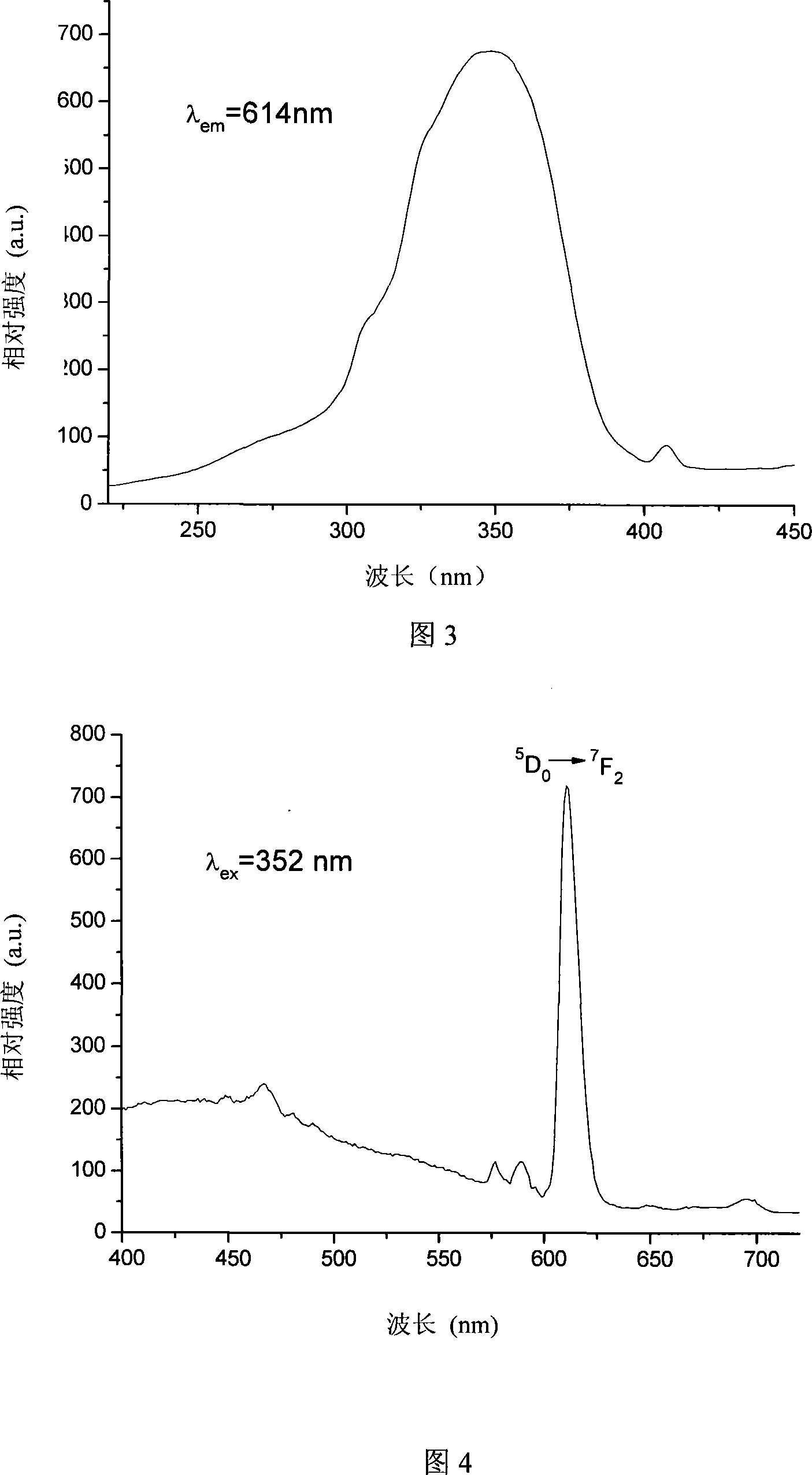
Process for producing beta-diketone functionalization rare earth mesoporous hybridisation luminescent material
A luminescent material and functionalized technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of low doping amount of rare earth complexes, uneven distribution, unstable rare earth complexes, etc. Insufficient stability, simple process, regular and orderly microstructure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of dibenzoylmethane intermediate
[0027] Dissolve 1 mmol of dibenzoylmethane in 20 mL of anhydrous tetrahydrofuran and add 2 mmol of NaH to the solution system. The reaction solution bubbles violently. After one and a half hours, add 2.2 mmol of propyltriethoxysilyl The tetrahydrofuran solution of propyl isocyanate was reacted at 65°C for 12 hours, and the solvent was removed by rotary evaporation to obtain an unusually viscous yellow oil drop product.
[0028] (2) Synthesis of dibenzoylmethane-functionalized binary mesoporous hybrid luminescent materials
[0029] First, weigh 1.0 g of Pluronic P123 surfactant, add 7.5 g of deionized water and 30 g of 2 mol / L hydrochloric acid, heat and stir to dissolve it. At room temperature, a mixture of TEOS and dibenzoylmethane intermediate was slowly added dropwise, with a molar ratio of 0.94:0.06, and stirred for 24 hours. Then put it into a stainless steel reaction kettle lined with polytetrafluoroethylene, and ...
Embodiment 2
[0031] (1) Synthesis of dibenzoylmethane intermediate
[0032] Dissolve 1.5 mmol of dibenzoylmethane in 25 mL of anhydrous tetrahydrofuran and add 3 mmol of NaH to the solution system. The reaction solution bubbles violently. After about one and a half hours, add 3.3 mmol of propyltriethoxy The tetrahydrofuran solution of propyl silyl isocyanate was reacted at 70°C for 12 hours, and the solvent was removed by rotary evaporation to obtain an unusually viscous yellow oil drop product.
[0033] (2) Synthesis of dibenzoylmethane-functionalized ternary mesoporous hybrid luminescent materials
[0034] Weigh 1.5g of PluronicP123 surfactant, add 11g of deionized water and 45g of 2mol / L hydrochloric acid, heat and stir to dissolve it. At room temperature, a mixture of TEOS and dibenzoylmethane intermediate was slowly added dropwise, with a molar ratio of 0.96:0.04, and stirred for 24 hours. Then put it into a stainless steel reaction kettle lined with polytetrafluoroethylene, and cry...
Embodiment 3
[0036] (1) Synthesis of acetylacetone intermediate
[0037] Dissolve 1mmol acetylacetone in 15mL anhydrous pyridine and add 2mmolNaH to the solution system. The reaction solution bubbles violently. After about one and a half hours, add 2.2mmol propyl triethoxysilyl isocyanate dropwise to the above reflux solution The tetrahydrofuran solution of propyl ester was reacted at 65°C for 12 hours, and the solvent was removed by rotary evaporation to obtain an unusually viscous yellow oil drop-like product.
[0038] (2) Synthesis of acetylacetone-functionalized mesoporous hybrid luminescent materials
[0039]Weigh 1.2g of PluronicP123 surfactant, add 9.0g of deionized water and 36g of 2mol / L hydrochloric acid, heat and stir to dissolve it. At room temperature, a mixture of TEOS and acetylacetone intermediate was slowly added dropwise, with a molar ratio of 0.94:0.06, and stirred for 24 hours. Then put it into a stainless steel reaction kettle lined with polytetrafluoroethylene, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com