Hyaluronic acid oligosaccharide fractions and drugs containing the same
A technology of hyaluronic acid and hyaluronic acid, applied in the field of hyaluronic acid oligosaccharide and its new fractions, can solve the problem of no explanation or hint and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
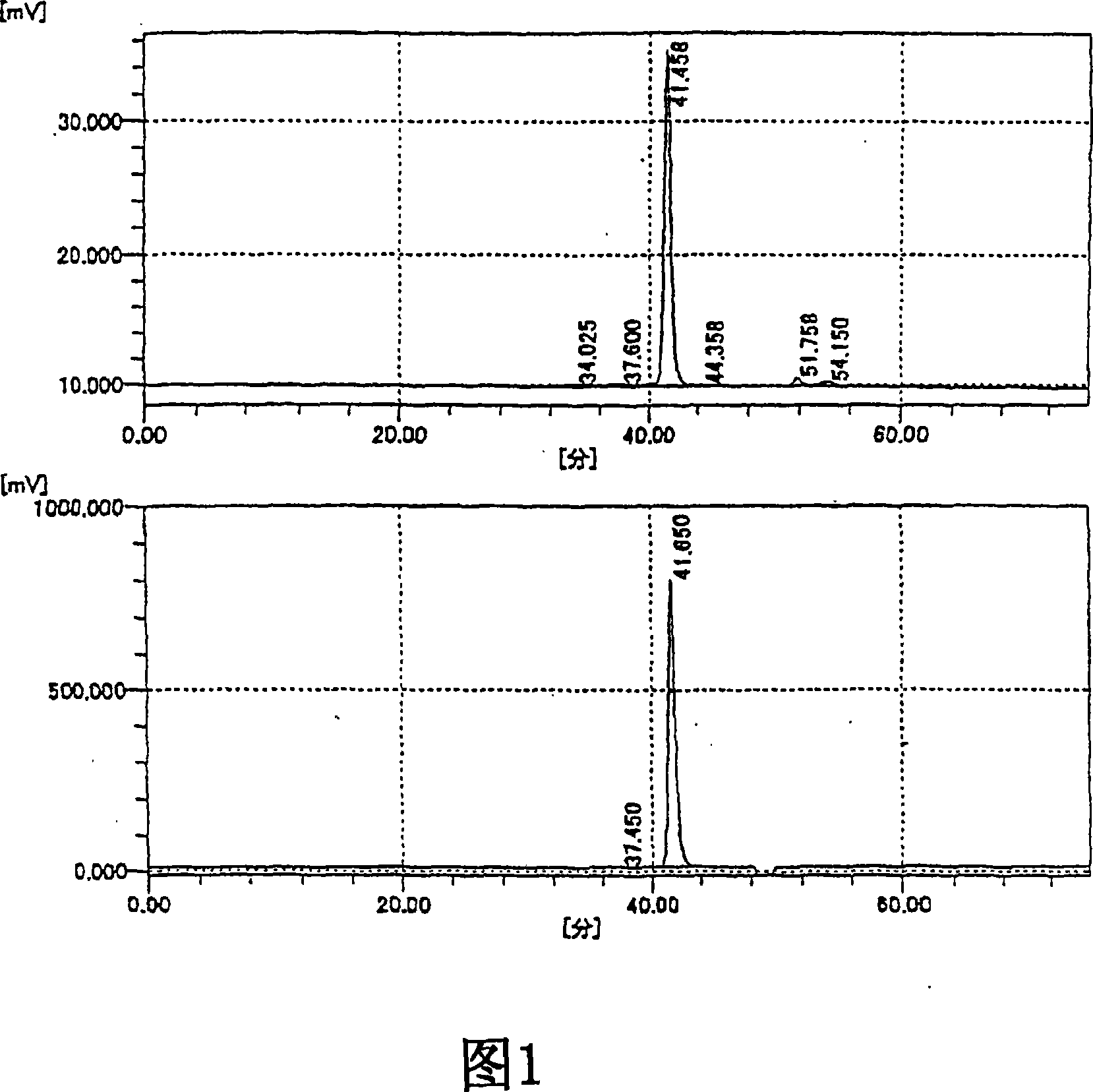
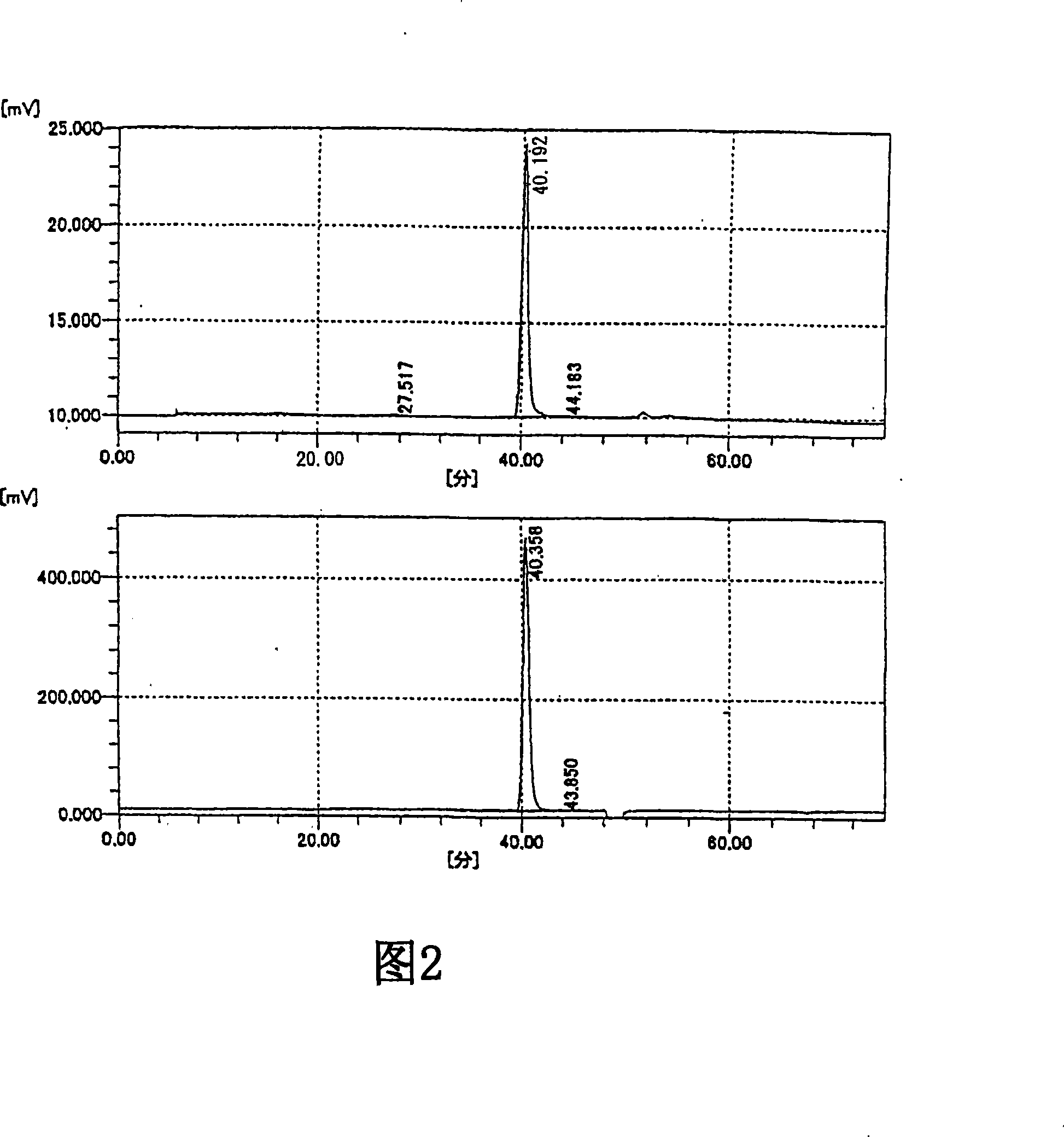
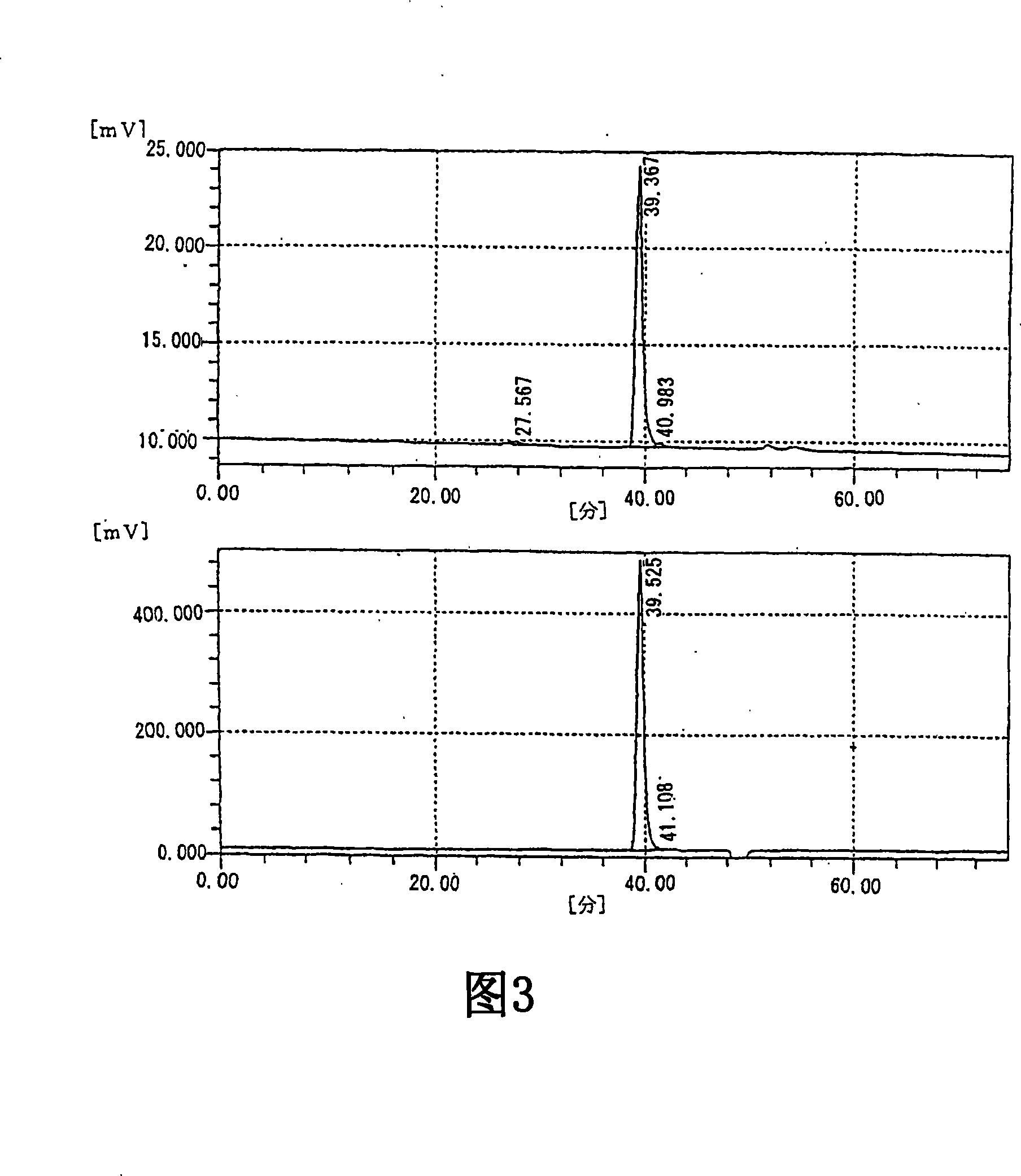
[0213] Preparation and physicochemical properties of oligosaccharides of the present invention and fractions of the present invention
[0214] 1. The preparation of oligosaccharide of the present invention and fraction of the present invention
[0215] The oligosaccharide of the present invention and the fraction of the present invention were prepared using the sodium salt of HA isolated and purified from cockscomb as a raw material by the following method. In addition, the sodium salt of HA as a raw material showed a single band by electrophoresis using a cellulose acetate membrane (electrophoresis buffer: pyridine-formic acid buffer, current: 15 mA, electrophoresis time: 30 minutes), and HA was not detected Other mucopolysaccharides (chondroitin, 4-chondroitin sulfate, 6-chondroitin sulfate, chondroitin sulfate E, chondroitin sulfate D, heparin, heparan sulfate, dermatan sulfate).
preparation example 1
[0216] (Preparation Example 1) Decomposition by Hyaluronidase
[0217] Dissolve 25 g of sodium salt of HA in 1.IL of 0.1 M phosphate buffer (pH 5.3) containing 0.15 M NaCl. To the obtained solution, 200 mg of hyaluronidase (5.342 units / mg; manufactured by Seikagaku Kogyo Co., Ltd.) derived from bovine testis was added and reacted at 37° C. for 9 hours.
[0218] After the reaction, the supernatant was collected by centrifugation at 10,000 rpm for 30 minutes. The recovered supernatant was loaded onto a Dowex 1×2 (100-200 mesh) (manufactured by Dow chemical) ion exchange column (φ1. 5×123cm), eluted by a linear concentration gradient of NaCl (0.01M~0.50M). Fractions containing HA oligosaccharides were screened by detecting uronic acid in the obtained fractions by the carbazole method. Appropriate fractions were pooled and concentrated, desalted using Sephadex G-10 (manufactured by Pharmacia; φ3×124), and then lyophilized.
[0219] The lyophilized weights of the obtained fract...
preparation example 2
[0221] (Preparation Example 2) Decomposition of ACI by Chondroitinase
[0222] Dissolve 8 g of sodium salt of HA in 500 ml of 0.1 M acetic acid buffer (pH6.0) containing 0.1% bovine serum albumin.
[0223] To the obtained solution, 32 units of chondroitinase AC I (manufactured by Seikagaku Kogyo Co., Ltd.) was added and reacted at 37° C. for 6 hours.
[0224] After the reaction, the supernatant was collected by centrifugation at 10,000 rpm for 30 minutes. The recovered supernatant was loaded onto a Dowex 1×2 (100-200 mesh) (manufactured by Dow chemical) ion-exchange column (φ4.5×123 cm), washed by a linear concentration gradient (0.01M-0.50M) of NaCl take off. Fractions containing HA oligosaccharides were screened by detecting uronic acid in the obtained fractions by the carbazole method. Appropriate fractions were pooled, concentrated, desalted with Sephadex G-10 (manufactured by Pharmacia; φ3×124), and then lyophilized.
[0225] The lyophilized weights of the obtained fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com