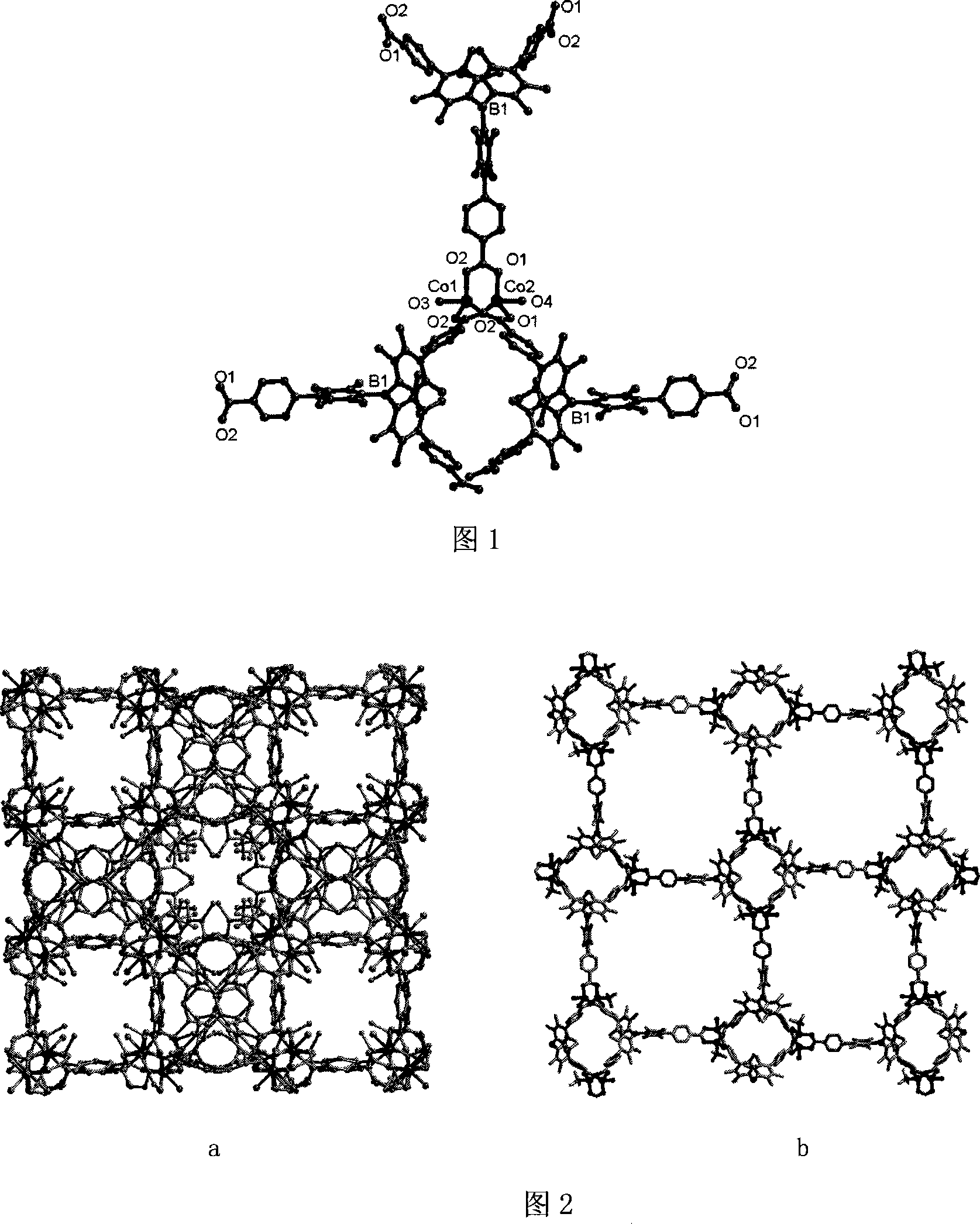
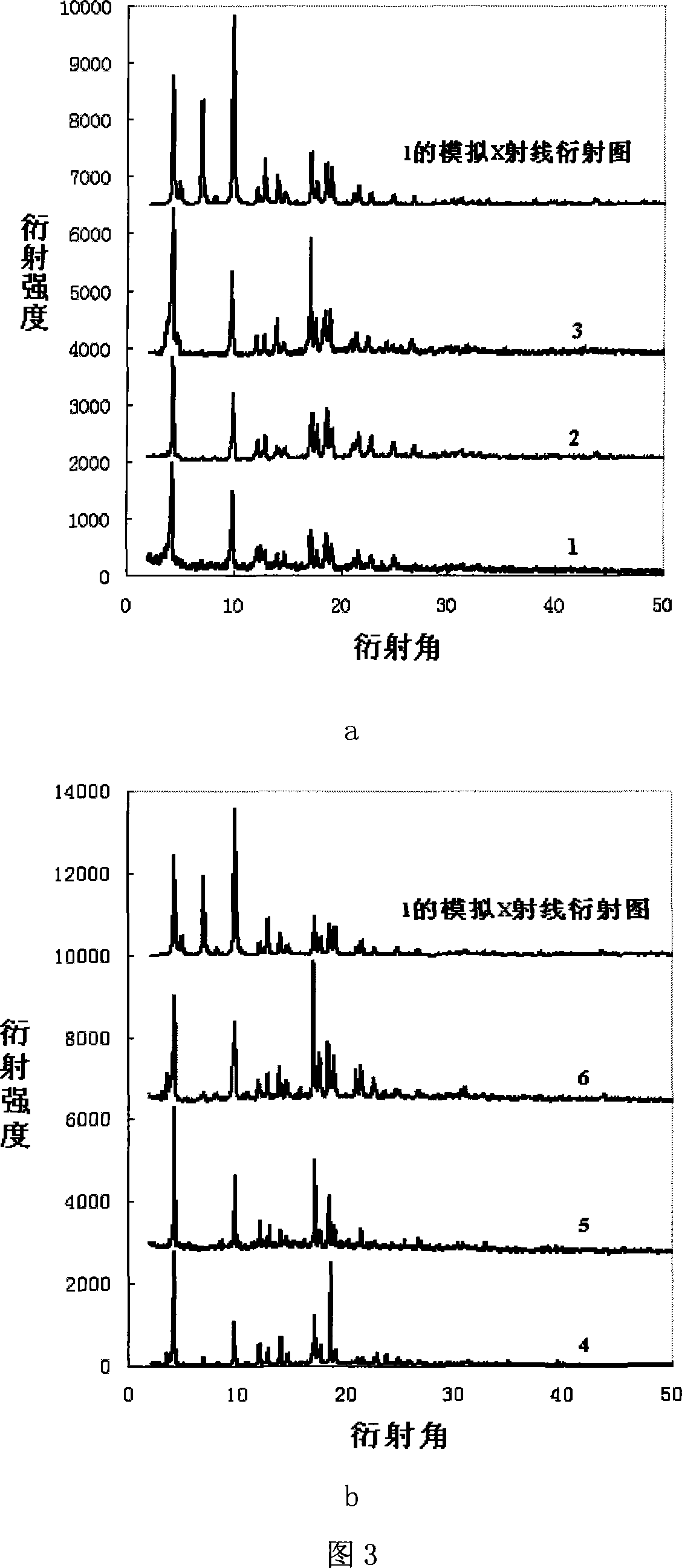
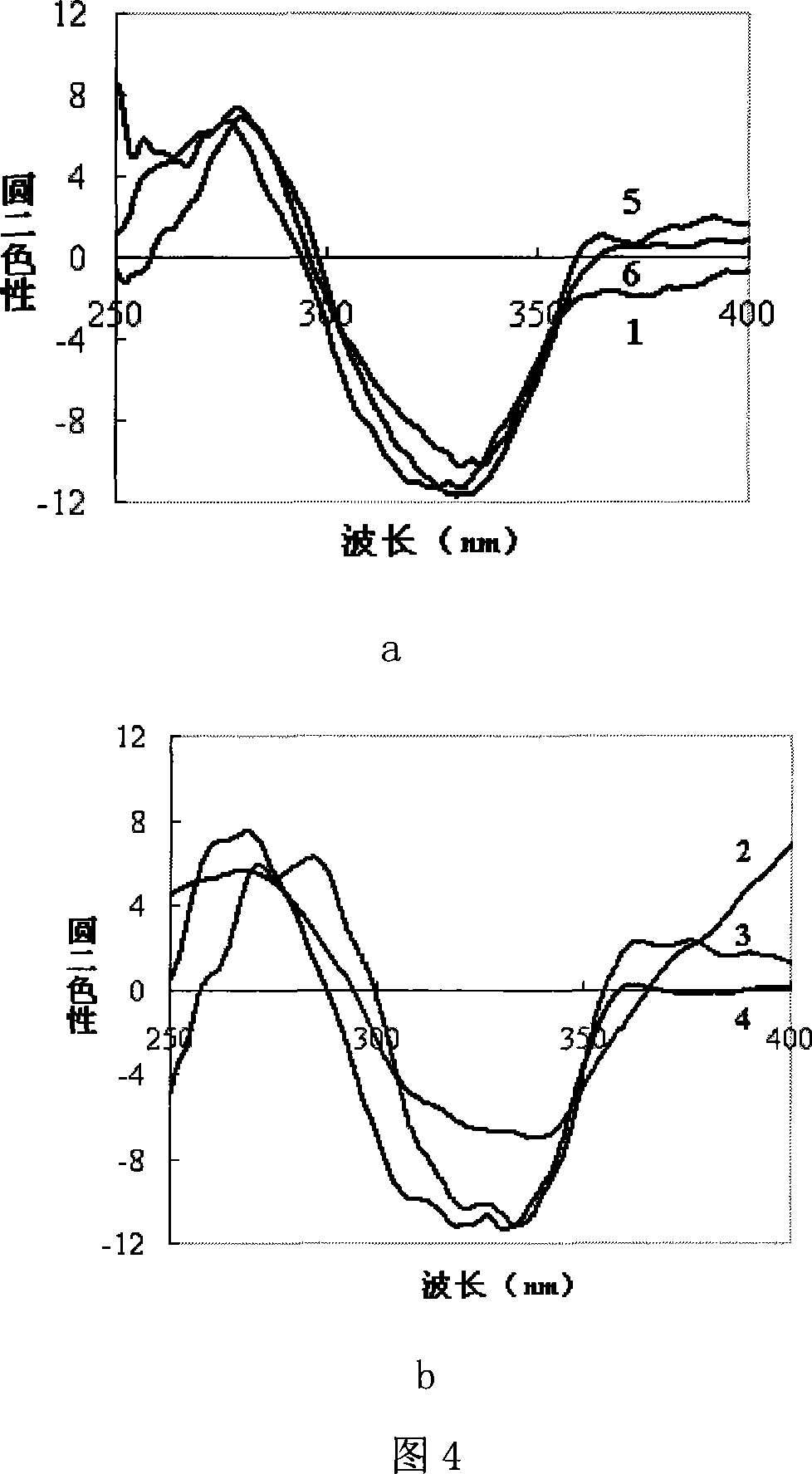
Method for preparing chirality non-linear optical metal-organic boron polymer crystal material
A technology of nonlinear optics and crystal materials, applied in the direction of zinc organic compounds, cadmium organic compounds, copper organic compounds, etc., can solve the problems of expensive, non-chiral, weak nonlinear optical effects, etc. Effect of simple, good second-order nonlinear optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] 1,2,4,5-Tetramethylbenzene (10g, 74.6mmol) and iodine (0.394g, 1.56mmol) were dissolved in 380 ml of dichloromethane, and slowly dripped into the light-shielding reactor under nitrogen protection Add bromine (9ml, 175.6mmol) in 40ml of dichloromethane solution (not less than 30 minutes), and reflux for 1 hour after the dropwise addition is complete. After the reaction was completed and returned to room temperature, 20 ml of 5M sodium hydroxide solution was added to quench the reaction. After liquid separation, the organic phase was washed several times with water and dried over anhydrous magnesium sulfate. After drying, the desiccant was filtered off, spin-dried to obtain a white solid, and then the white needle-shaped target product 1,4-dibromo-2,3,5,6-tetramethylbenzene 16.76g (yield : 77%).
Embodiment 2
[0030]
[0031] Under the protection of nitrogen, 1,4-dibromo-2,3,5,6-tetramethylbenzene (10 g, 34.3 mmol) was dissolved in 300 ml of dry ether, and added dropwise to the reaction solution at -78°C Pentane solution of n-butyllithium (2.5M, 13.6ml, 34mmol), after the dropwise addition, allow the reaction solution to slowly return to 0°C, stir at 0°C for 20 minutes, then re-warm to -78°C, slowly Add boron trifluoride·diethyl ether (1.4ml, 11.2mmol) dropwise, return to room temperature slowly (not less than 1 hour) after dropping, and stir at room temperature for 16 hours. After the reaction, add water, separate the liquids, extract the aqueous phase with ether, wash the combined organic phases with brine several times, dry over anhydrous magnesium sulfate, filter and spin dry after drying, and recrystallize with ether / methanol mixed solvent to obtain the target product three- (4-Bromo-2,3,5,6-tetramethylphenyl)borane 5.5 g (yield: 75%).
Embodiment 3
[0033]
[0034] Under the protection of nitrogen, slowly dropwise added tri-(4-bromo-2,3,5,6-tetramethylbenzene)borane (5.23g, 8.1mmol) in THF solution (200ml) at -78°C Pentane solution of tert-butyllithium (1.5M, 33ml, 49.3mmol), after the dropwise addition was completed, react at a low temperature of -78°C for 50 minutes, then add 80ml of a tetrahydrofuran solution of iodine (9.4g, 37.0mmol) dropwise at -78°C After dropping, slowly return to room temperature (not less than 1 hour), and stir at room temperature for 11 hours. After the reaction, concentrate and spin off most of the solvent, add water and extract with ether, the combined organic phase is washed with sodium thiosulfate solution and brine, dried with anhydrous magnesium sulfate, filtered after drying, and spin-dried, the crude product is extracted with ether / methanol Recrystallization from a mixed solvent gave 4.9 g of the high-purity target product tris-(4-iodo-2,3,5,6-tetramethylbenzene)borane (yield: 77%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com