Liver target anticancer nano prodrug system based on tree shaped polymer, preparation and use
A polymer and liver-targeted technology, applied in the fields of biomedical technology and nanomedicine, can solve the problems of low selectivity of chemical drugs and radiopharmaceuticals, fast metabolism of small molecule drugs, cytotoxic side effects, etc., to improve clinical efficacy and Bioavailability, overcoming the unsatisfactory clinical treatment effect, enhancing the effect of phagocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Synthesis of HO-PEG-PAMAM:
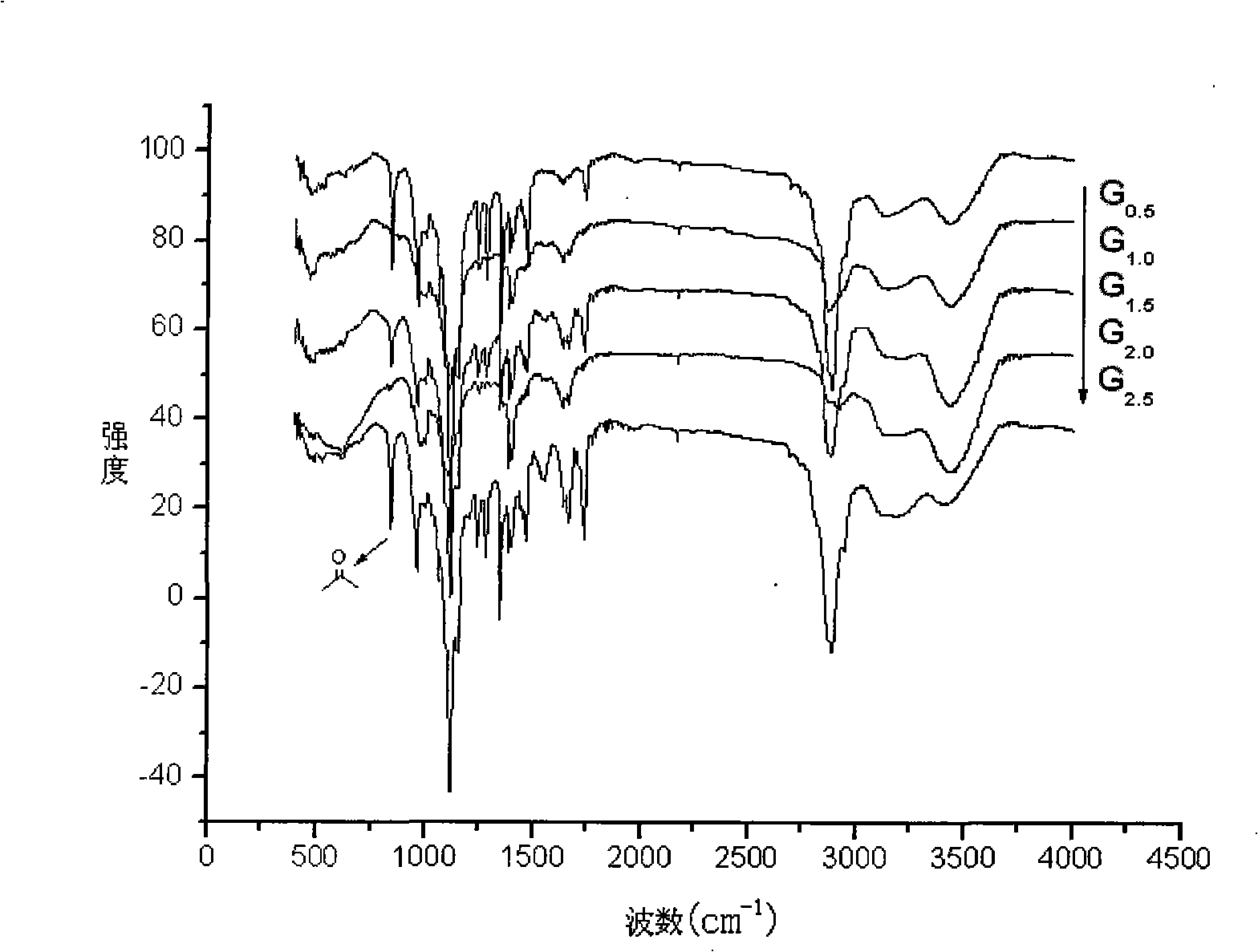
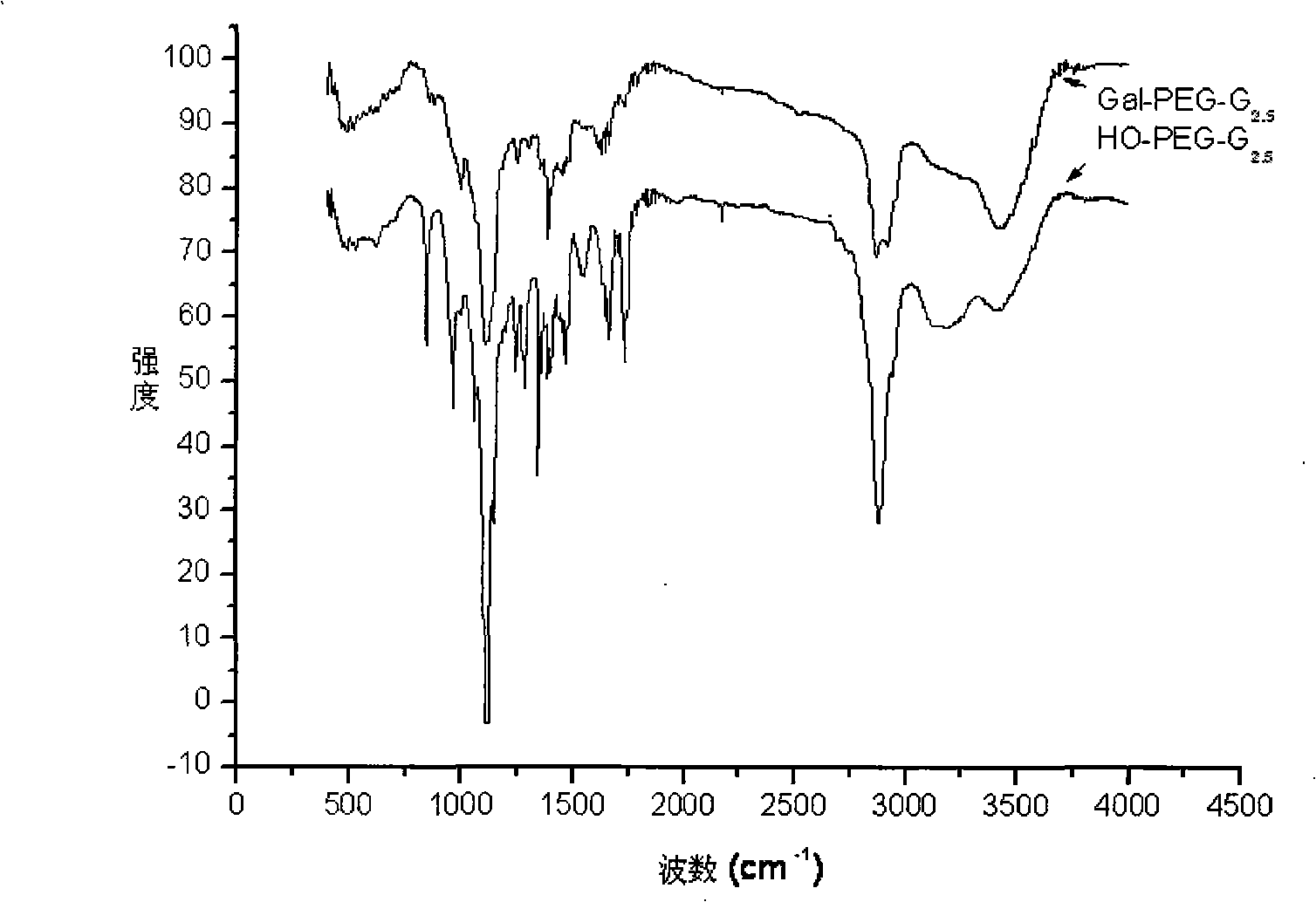
[0043] 0.1732g (0.0507mmol) of monosubstituted polyethylene glycol amine (HO-PEG-NH 2 ) was dissolved in anhydrous methanol, 0.913ml (10.1mmol) methyl acrylate (MA) was added, reacted in the dark at 25°C for 72 hours, most of the solvent was evaporated by rotary evaporation, and then dissolved in a small amount of methanol, and dissolved in a large amount of anhydrous ether Re-precipitated, centrifuged to separate the precipitate and vacuum-dried to obtain half generation 0.5 generation (G 0.5 ); then G 0.5 Dissolve in anhydrous methanol, add 1.352ml (20.2mmol) ethylenediamine (EDA), react at 25°C for 72 hours, evaporate most of the solvent by rotary evaporation, then dissolve in a small amount of methanol, and reprecipitate in a large amount of anhydrous ether, The precipitate was separated by centrifugation and vacuum-dried to obtain generation 1.0 (G 1.0 ); then the G 1.0 Dissolve in anhydrous methanol, add 3.651ml (40.4mmol) MA, r...
Embodiment 2
[0048] Example 2: NH 2 -PEG-NH 2 Synthesis
[0049] PEG→Cl-PEG-Cl
[0050] Take PEG-1000 (10.3g, 10.3mmol) and dissolve it in toluene (250mL), and use an oil-water separator to azeotropically remove water. Add freshly distilled pyridine (0.83mL, 10.3mmol) and thionyl chloride (7.3mL, 103mmol), and reflux at 75°C for 12h. Cool to room temperature, remove most of the solvent by rotary evaporation, redissolve in dichloromethane, and use Dry the water with potassium carbonate for 12 hours, filter, and then absorb the filtrate with neutral aluminum oxide (20g, activated for 2 hours at 120°C), filter, concentrate the filtrate, precipitate it with ether, dissolve it in dichloromethane, and reprecipitate once with ether , centrifuged, and vacuum-dried.
[0051] Cl-PEG-Cl→N 3 -PEG-N 3
[0052] Dissolve Cl-PEG-Cl (7g, 6.8mmol) in DMF (20mL), add sodium azide (7.7g, 118mmol), and stir at reflux at 70°C for 24h. The reaction solution was cooled to room temperature and then filtere...
Embodiment 3
[0057] Example 3: T-PEG-NH 2 Galactosyl polyethylene glycol amine Gal-PEG-NH whose T targeting group is galactosyl 2 Synthesis
[0058] 1. Mono Boc protected polyethylene glycol diamine (Boc-PEG-NH 2 )Synthesis
[0059] Take 2.0922g (2.0922mmol) NH 2 -PEG-NH 2 Dissolve in 50mL CH 2 Cl 2 , then 0.4812mL (2.0922mmol) di-tert-butyl dicarbonate (Boc anhydride) and 0.6956mL (4.8121mL) triethylamine (Et 3 N) dissolved in 50mL CH 2 Cl 2 Add the above NH dropwise under nitrogen protection at 30°C 2 -PEG-NH 2 Solution, after 48 hours of reaction, rotary evaporation removed most of the solvent, and then dissolved in a small amount of CH 2 Cl 2 and reprecipitated in ether, centrifuged and vacuum-dried the solid, and then separated and purified by silica gel column chromatography, the eluent was CHCl 3 / CH 3 OH, the ratio is 16 / 1. The product was dried in vacuo after most of the solvent was removed by rotary evaporation.
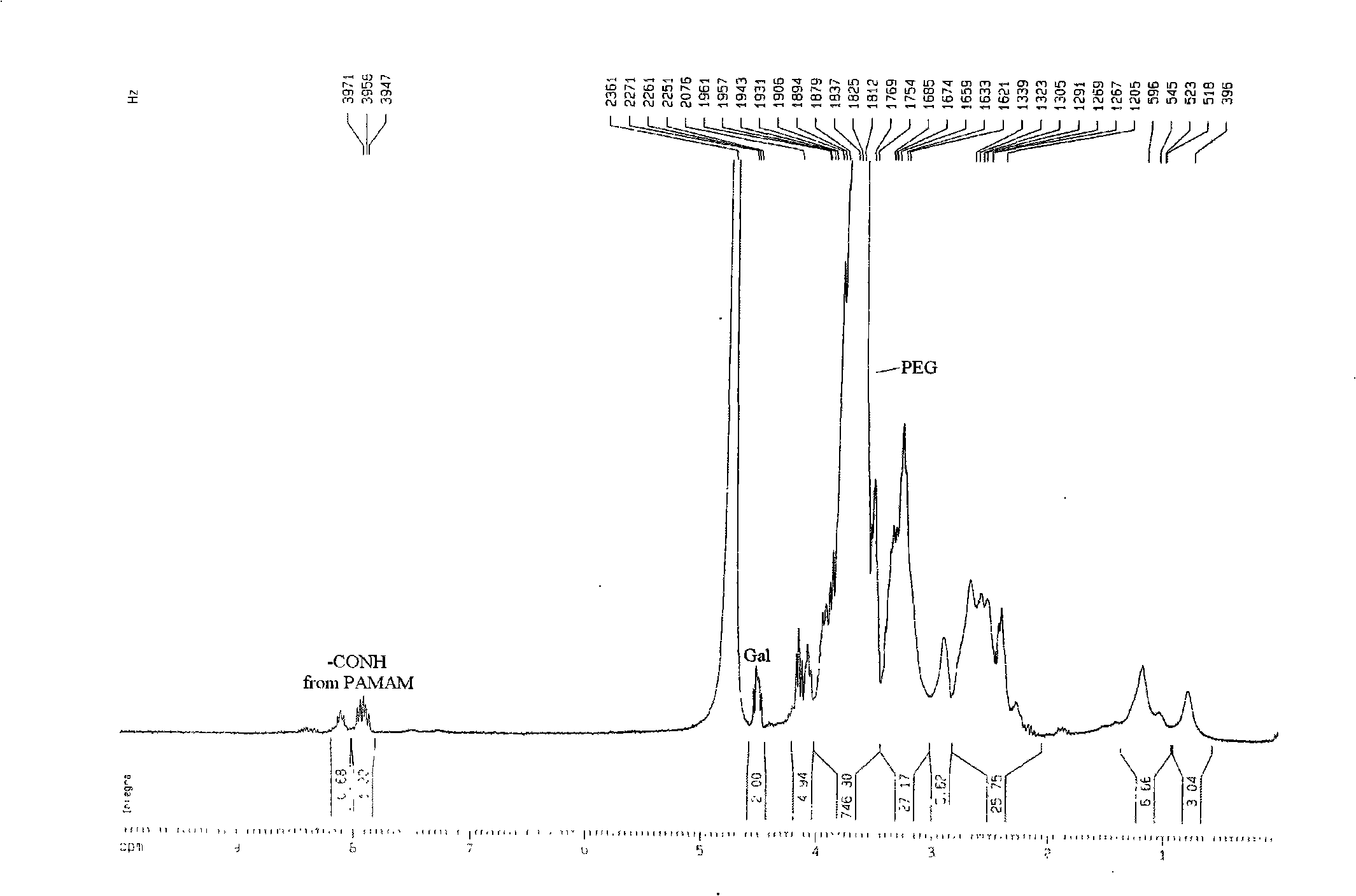
[0060] 2 Galactosyl polyethylene glycol amine (Gal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com