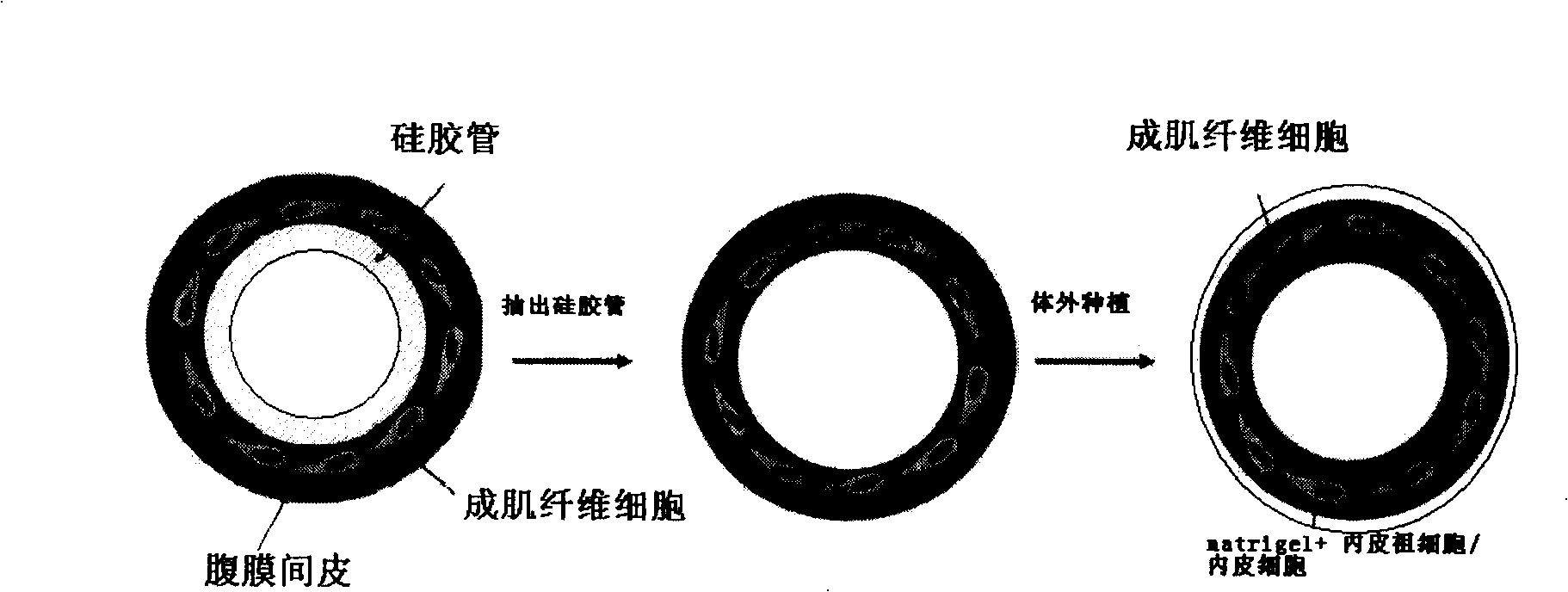
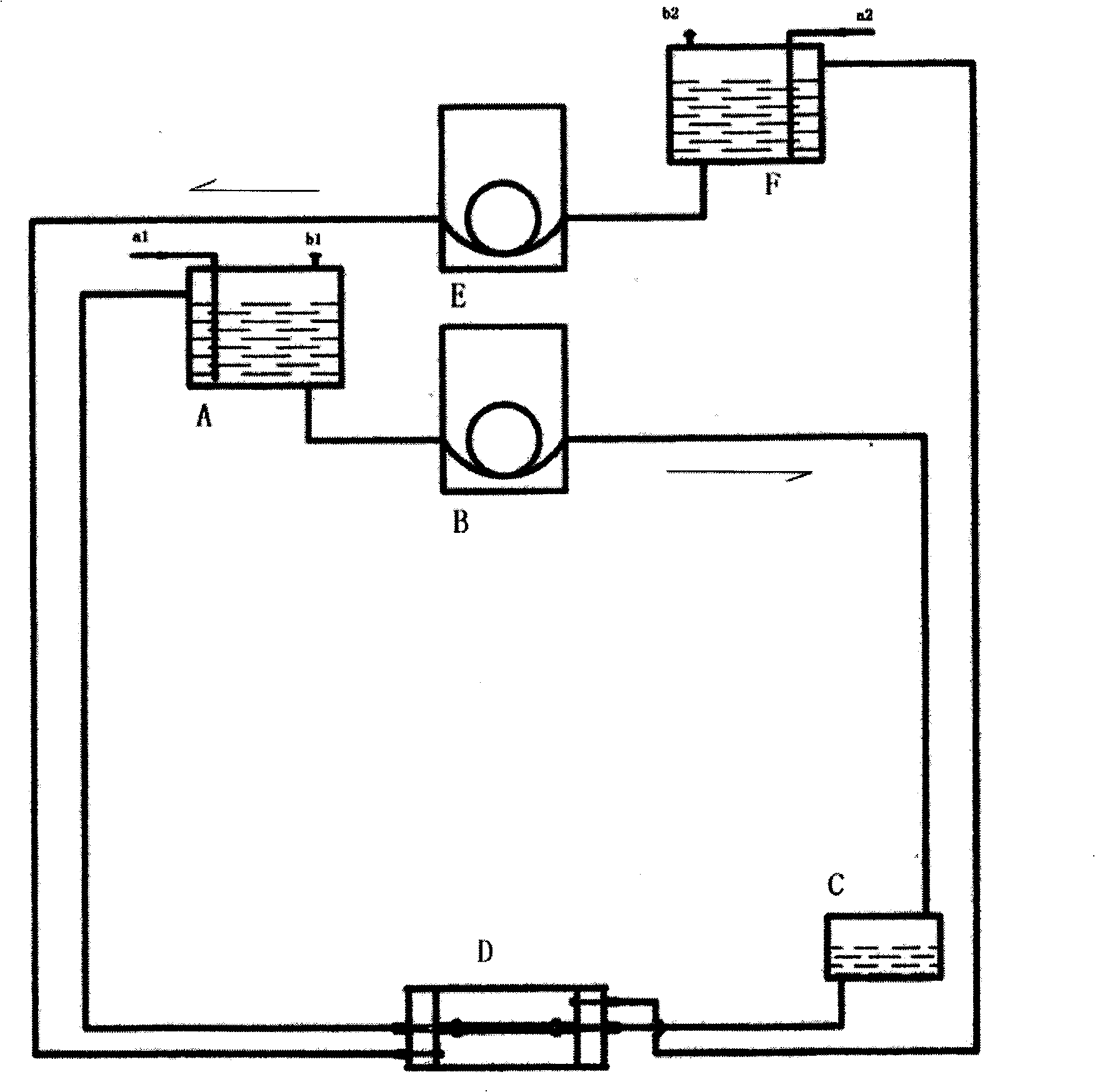
Construction method of tissue engineering blood vessel
A tissue engineering and construction method technology, applied in the construction of tissue engineered blood vessels, in the field of in vitro construction of vascular grafts with autologous living tissue materials, can solve the problems of mechanical properties, compliance restricting wide application, infectious diseases, antigen residues, etc. Effects of immune rejection and inflammatory response, perfect cytocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Reagents and materials
[0050] 1. Phosphate buffer saline (PBS solution): a small packet of commercially available PBS powder (Gibco), dissolved in 1000ml of distilled water, adjusted to pH 7.2, filtered and sterilized, aliquoted, and placed at 4°C for later use.
[0051] 2. M199 culture medium: a small bag of commercially available M199 powder (Gibco), add 1.2g of sodium bicarbonate, 1.5mol / LHEPES 10ml, three distilled water to 1L, adjust the pH to 7.2, filter and sterilize, and pack in 250ml, Store at 4°C for later use.
[0052] 3. DMEM culture medium: a small bag of commercially available DMEM powder (Gibco), add 1.2g of sodium bicarbonate, 1.5mol / LHEPES 10ml, three distilled water to 1L, adjust the pH to 7.2, filter and sterilize, pack in 250ml, Store at 4°C for later use.
[0053] 4. Endothelial cells and internal circulation culture medium: M199 culture medium containing 15-20% FBS and 0.1 mg / ml endothelial cell growth supplement (ECGS, purchased from Instit...
Embodiment 2
[0069] Example 2. Results evaluation
[0070] Specimens were collected from the fresh autologous living tissue biological tubes and tissue engineered blood vessels involved in Example 1, and mechanical measurements and biological tests were carried out according to the following methods.
[0071] 1. Method
[0072] (1) Mechanical testing
[0073] 1. Determination of tensile strength at break: cut the specimen into a plate shape, and break it on a tensile machine. The measured maximum tensile force is divided by the cross-sectional area of the experimental material, which is the material tensile strength of the specimen, indicating the self-activity. Stretch resistance of tissue materials.
[0074] 2. Determination of elongation at break: Cut the specimen into a plate shape, break it on a tensile machine, record the change in length of the sample from being stretched to being broken, and divide it by the original length of the sample, the resulting percentage is The elonga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com