Reactivity high-branching vinyl polymer and preparation method
A vinyl compound and branched ethylene technology, applied in the field of reactive hyperbranched polymers and their preparation, can solve the problems such as the inability to directly use vinyl compounds, and achieve the effects of wide optional range and various combinations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: emulsion polymerization (E)
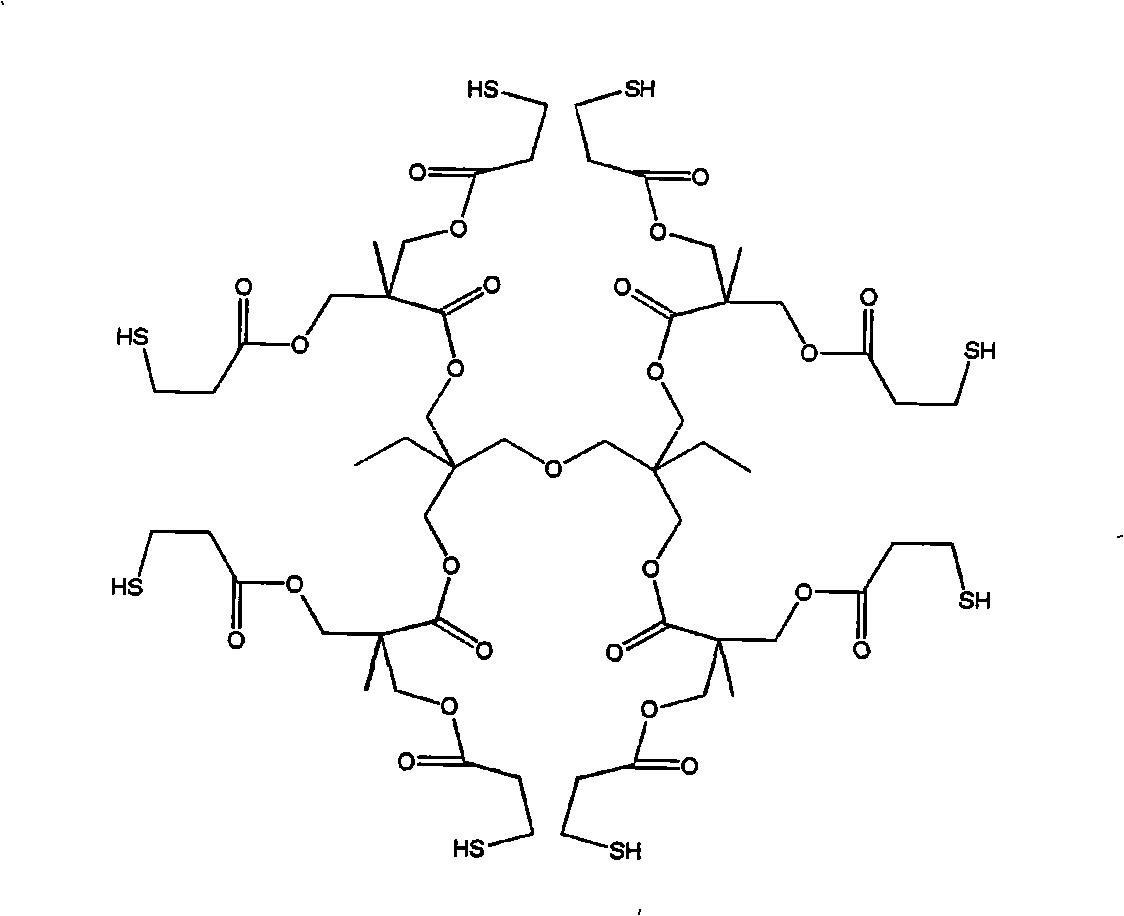
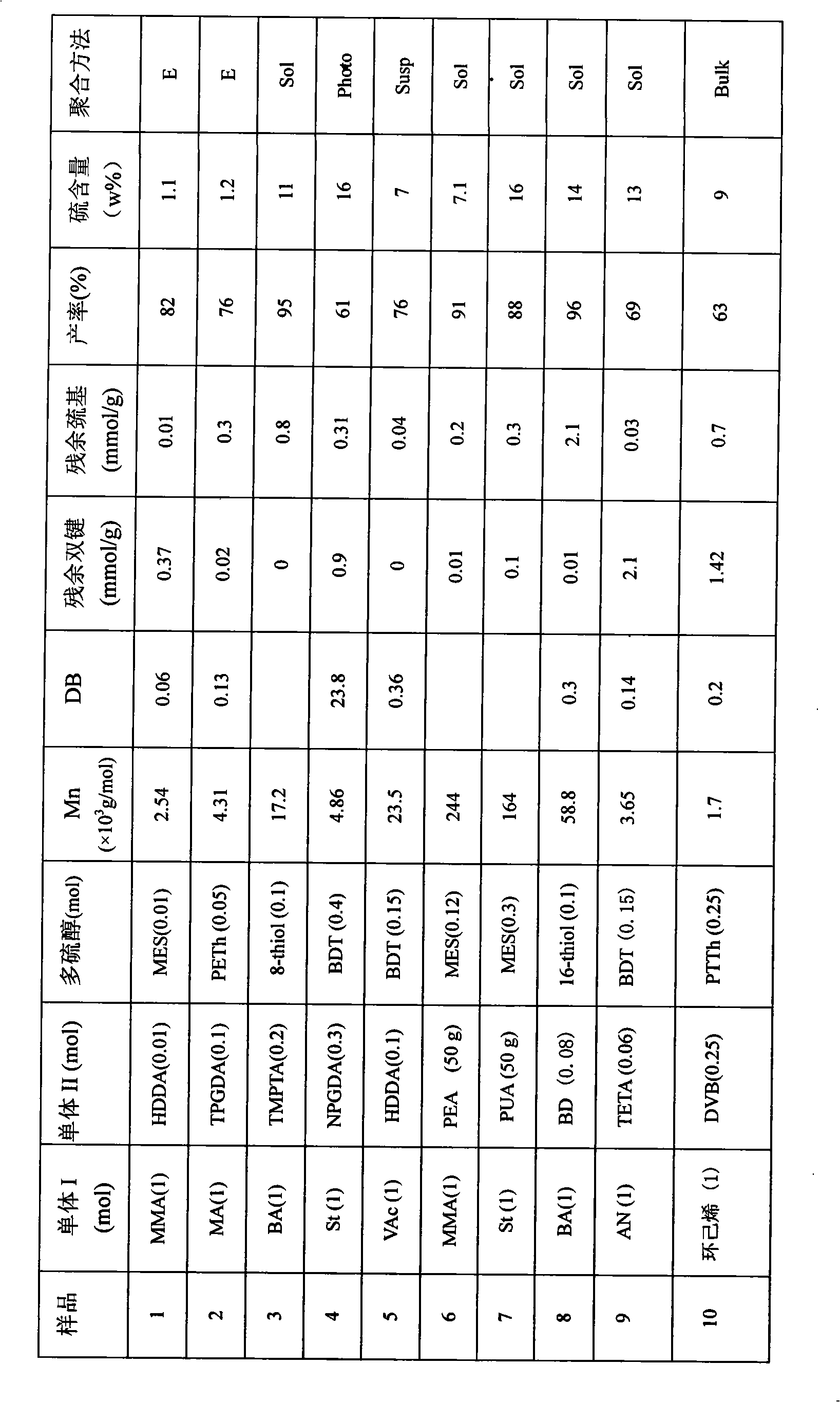
[0027] 5 grams of emulsifier sodium dodecylbenzenesulfonate, monofunctional compound MMA 100g (1mol), polyfunctional compound HDDA 2.3g, (0.01mmol), polythiol MES 1.54g (0.01mmol) and 100ml deionized water Add it into the reactor, stir and pre-emulsify for about 30 minutes under nitrogen gas, add 4 g of potassium persulfate initiator, move the reactor into a heating water bath, keep stirring, and react at about 68 ° C for about 3 hours with nitrogen gas to stop. The product is demulsified by freezing, and the separated product is dissolved in an organic solvent and precipitated with heptane at low temperature. The dissolution-precipitation was repeated three times, and the solvent was removed in a vacuum oven at about 45°C to obtain a purified reactive hyperbranched vinyl polymer. Its chemical structure is shown in the No. 1 embodiment in the attached table 1.
Embodiment 2
[0028] Example 2: Solution Polymerization (Sol)
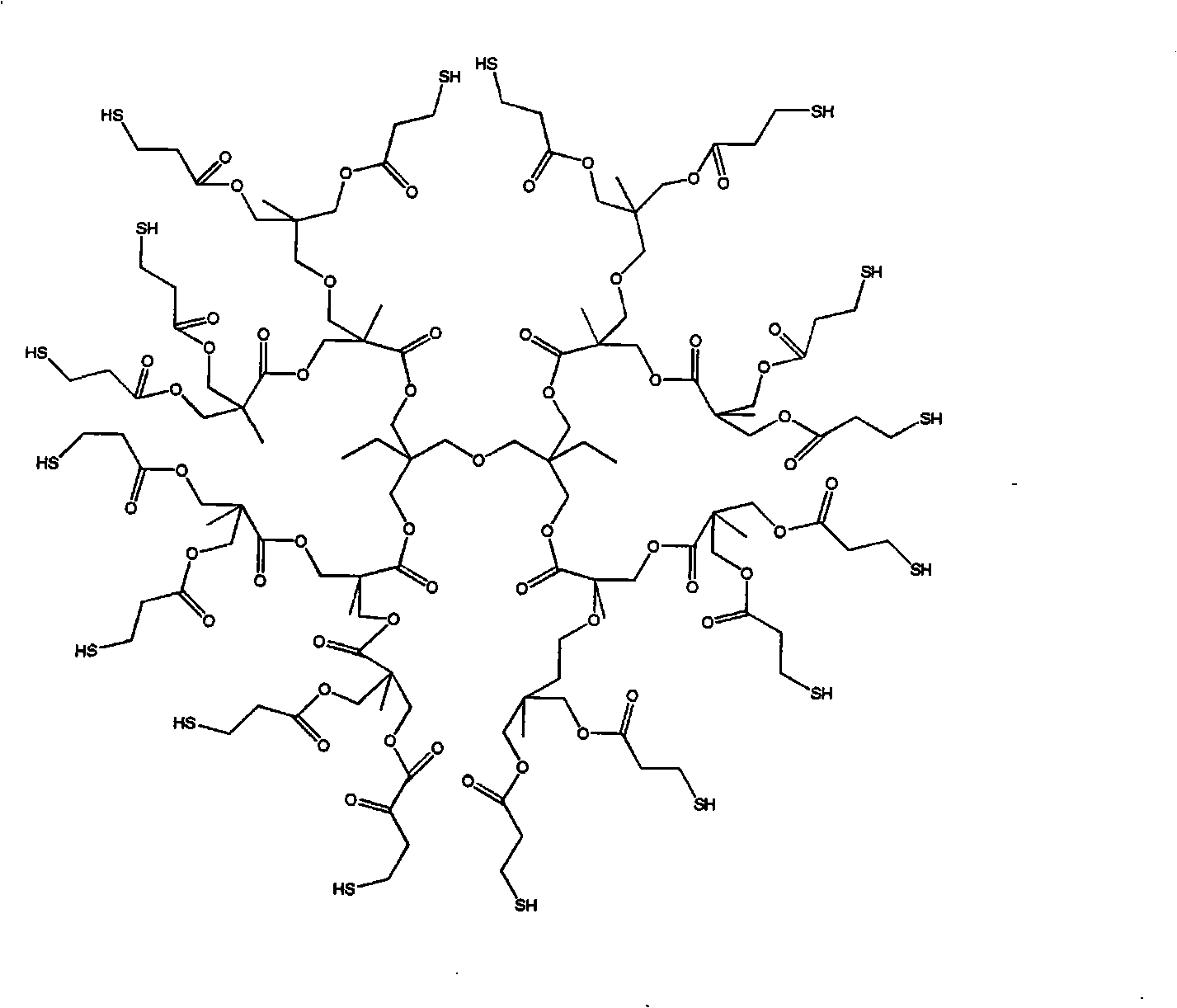
[0029] Add 104g of monofunctional compound St, 50g of polyfunctional compound PUA, 46gh of polythiol MES and 2g of initiator AIBN into the reactor, and then add 150ml of solvent toluene; pass in nitrogen, raise the temperature to about 70°C, and stir for about 4 hours ; The polymer solution was precipitated with heptane at low temperature. The dissolution-precipitation was repeated three times, and the solvent was removed in a vacuum oven at about 40°C to obtain a purified reactive hyperbranched vinyl polymer. Its chemical structure is shown in the No. 8 embodiment in the attached table 1.
[0030] Embodiment 3 suspension polymerization (Susp)
[0031] Add 0.2g of polyvinyl alcohol, 6g of magnesium carbonate, 86g of monofunctional compound VAc, 23g of polyfunctional compound HDDA, 18g of polythiol BDT, 5g of initiator azobisisoheptanonitrile and 220ml of deionized water into the reactor, After stirring and pre-emulsifying fo...
Embodiment 4
[0032] Embodiment 4 bulk polymerization (Bulk)
[0033] Add 80g of monofunctional compound cyclohexene, 32g of polyfunctional compound DVB, 50g of polythiol PTTh and 0.2g of initiator azobisisobutyronitrile into the reactor, blow nitrogen gas, and stir the reaction at (60-75)°C hours. The product was dissolved in an organic solvent and precipitated with heptane at low temperature. The dissolution-precipitation was repeated three times, and the solvent was removed in a vacuum oven at about 35°C to obtain a purified reactive hyperbranched vinyl polymer. Its chemical structure is shown in Example 10 of Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com