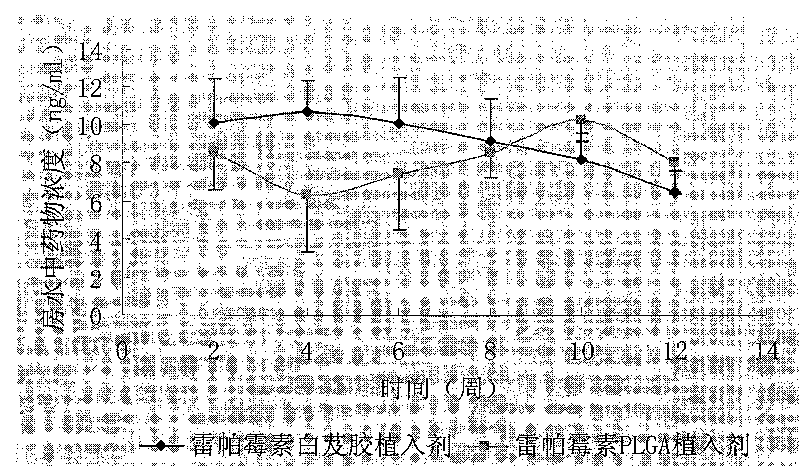
Rapamycin eye-in implantation type medicine release system with bletilla striata glue as carrier
A technology of rapamycin and bletilla jelly, which is applied in the field of intraocular implant drug delivery system, to achieve the effect of inhibiting new blood vessels, improving the effect of prevention and treatment, and reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The extraction of the bletilla striata gum of the present invention: extract and purify the bletilla striata gum required by the present invention with the following process.
[0026] 1. Crude polysaccharide extraction
[0027] The dried tuber products of Bletilla striata were crushed, sieved, dried, added 20 times the volume of distilled water, and extracted under reflux at 60°C for 4 hours. Repeat the extraction once. Combine the two extracts. Centrifuge (5000×g, 10min), take the supernatant, concentrate under reduced pressure at 70°C, add three volumes of 95% ethanol to precipitate. The precipitate was collected by centrifugation and freeze-dried to obtain crude bletilla striata gum (abbreviated as BR0).
[0028] 2. Separation and purification
[0029] Dissolve BR0 in the minimum volume of water, deproteinize it by Sevag method until almost no protein is detected by Coomassie brilliant blue G-250 method, then dialyze with convective water for three days, and vacu...
Embodiment 2
[0034] Select the Bletilla striata glue prepared in Example 1, prepare 12% bletilla striata glue aqueous solution, take 20.0ml of the bletilla striata glue aqueous solution, add it in a sterile glass bottle, add rapamycin micronized powder 600mg under aseptic conditions, stir and disperse evenly, Then add 20 mg of ethylene glycol diglycidyl ether, stir evenly, and place in a water bath at 55°C for 3-5 hours. Take it out and wash it sufficiently to remove the residual cross-linking agent, pour the cross-linked glue solution into a polytetrafluoroethylene mold, and place it in an oven at 40°C to dry to form a film. After the diaphragm is fully dried, take it out and put it into normal saline for injection to fully soak and wash it, take out the diaphragm, and after it is completely dry, obtain a sheet medicine with a thickness of 1.5-2 mm, and then stamp it with a die with an aperture of 1.5-2.0 mm Formulations with a thickness of 1.0-2.0 mm and a diameter of 1.5-2.0 mm. The pr...
Embodiment 3
[0036] In Example 1, ethylene glycol diglycidyl ether is replaced with propylene glycol diglycidyl ether, and the rest of the conditions are unchanged, and the same crosslinking effect can also be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com