Biological adsorbing agent with adsorption capacity of heavy metal ion, and producing method and application thereof
A technology of heavy metal ions and biological adsorbents, which is applied in the direction of adsorption of water/sewage treatment, other chemical processes, chemical instruments and methods, etc., can solve problems such as refractory degradation, secondary pollution, research literature and patent reports that have not been seen, and achieve Effects of high specific surface area and mechanical strength, good adsorption capacity, and good solvency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
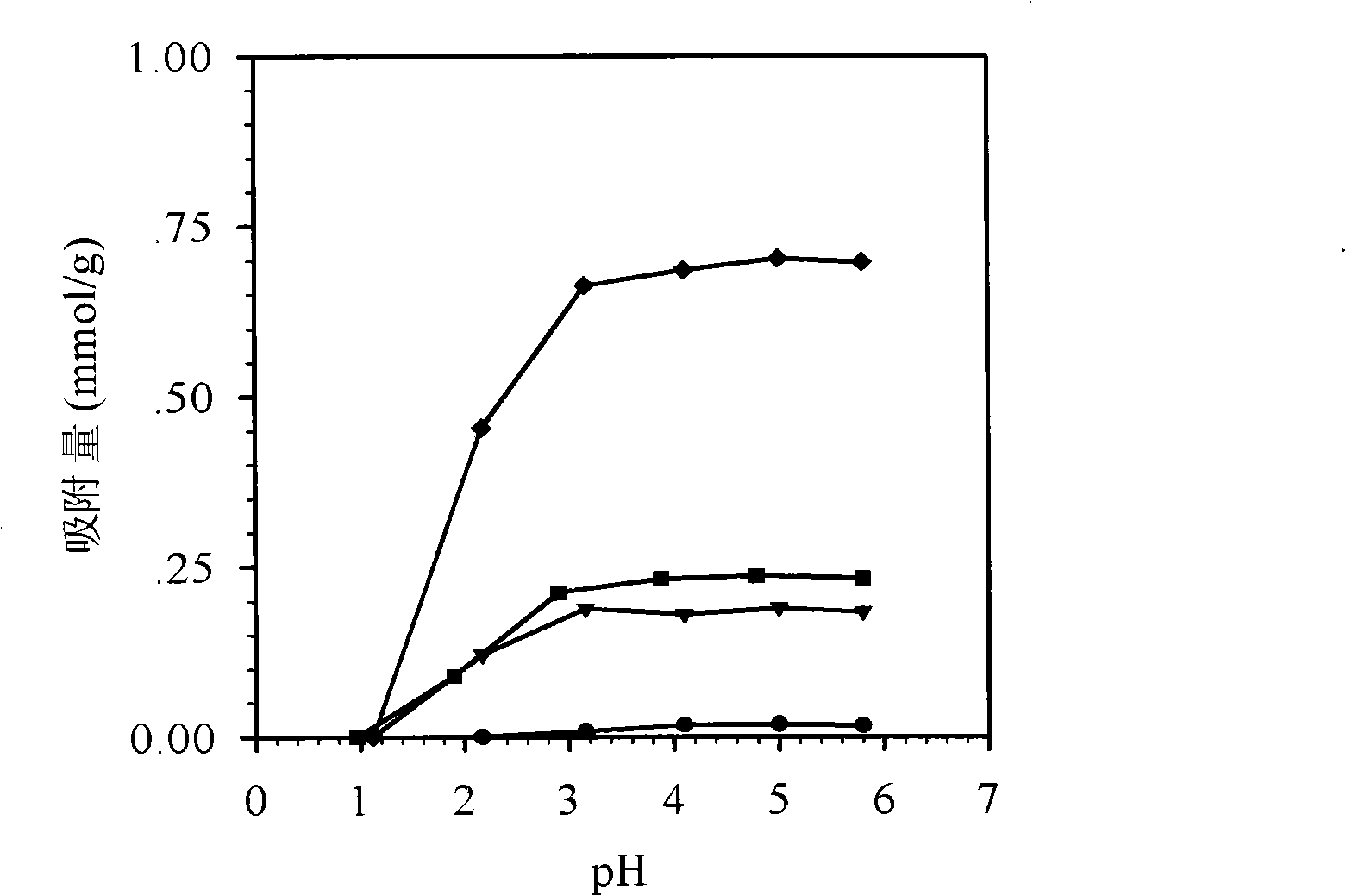
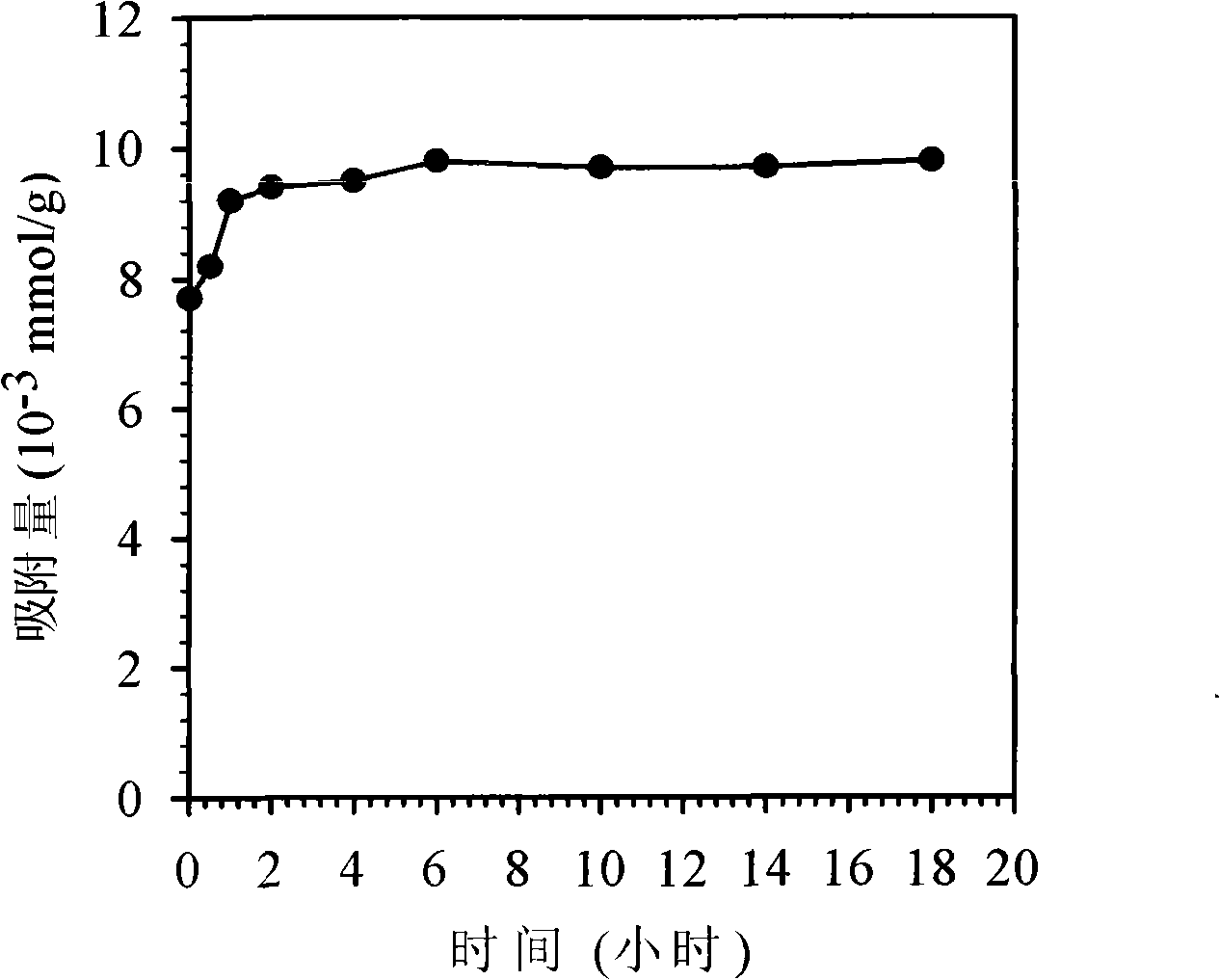
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation by drying method: chitin / cellulose adsorbent
[0036] Chitin and microcrystalline cellulose were dried in a vacuum desiccator at 70 °C for 24 hours. Under the microwave heating condition of P-3 power, chitin and microcrystalline cellulose with a mass ratio of 1:2 were dissolved in [Bmim][Cl ]middle. The resulting viscous, clear solution with a mass percentage of 6% was squeezed into beads in a water bath through a 1.6 mm syringe needle. Then the chitin / cellulose adsorbent was rinsed 5 times with deionized water. Determination of [Bmim] in the aqueous phase with a UV spectrophotometer at 211nm + , OK[Bmim] + It has all been washed. The obtained chitin / cellulose adsorbent was dried in an oven at 70°C for later use.
Embodiment 2
[0037] Example 2: Preparation by drying method: protonated chitin / cellulose adsorbent
[0038] Protonated chitin (chitin was soaked in 1 mol / L hydrochloric acid for 5 hours, washed with water until neutral, and dried in vacuum at 40°C for 24 hours) and microcrystalline cellulose were dried in a vacuum dryer at 70°C for 24 hours. Under the microwave heating condition of P-3 power, protonated chitin and microcrystalline cellulose with a mass ratio of 1:2 were dissolved in [Bmim] successively for 35 times (10S each time) and 25 times (8S each time). [Cl]. The resulting viscous, clear solution with a mass percentage of 6% was squeezed into beads in a water bath through a 1.6 mm syringe needle. The protonated chitin / cellulose adsorbent was then rinsed 5 times with deionized water. Determination of [Bmim] in the aqueous phase with a UV spectrophotometer at 211nm + , OK [Bmim] + It has all been washed. The obtained protonated chitin / cellulose adsorbent was dried in an oven at 70...
Embodiment 3
[0039] Embodiment 3: drying method preparation: chitosan / cellulose adsorbent
[0040] Chitosan and microcrystalline cellulose were dried in a vacuum desiccator at 70 °C for 24 hours. Under the microwave heating condition of P-3 power, chitin and microcrystalline cellulose with a mass ratio of 1:2 were dissolved in [Bmim][ Cl]. The resulting viscous, clear solution with a mass percentage of 6% was squeezed into beads in a water bath through a 1.6 mm syringe needle. The chitosan / cellulose adsorbent was then rinsed 5 times with deionized water. Determination of [Bmim] in the aqueous phase with a UV spectrophotometer at 211nm + , OK[Bmim] + It has all been washed. The obtained chitosan / cellulose adsorbent was freeze-dried in a lyophilizer, and then placed in an oven at 70° C. for subsequent use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com