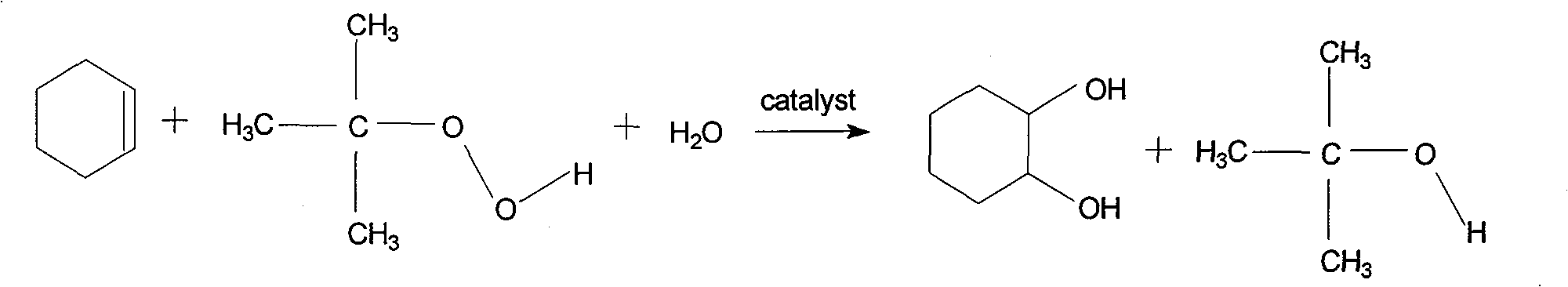
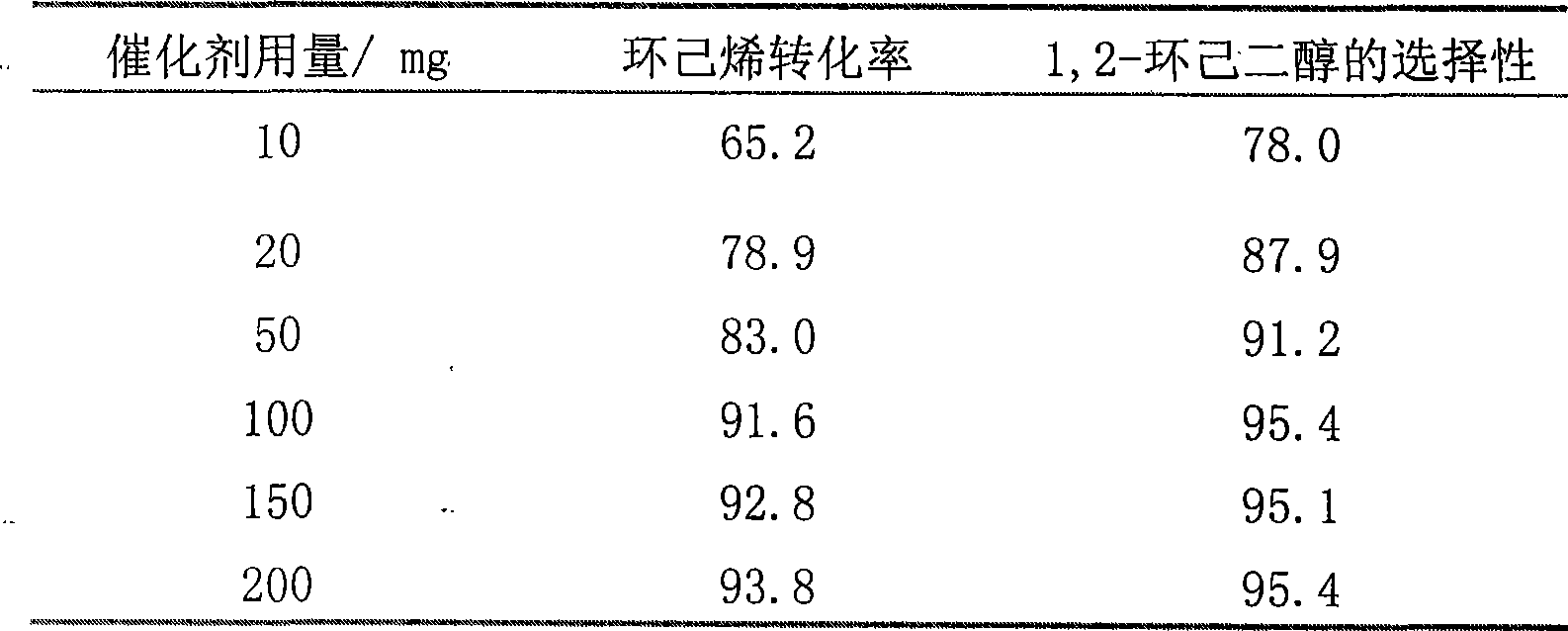
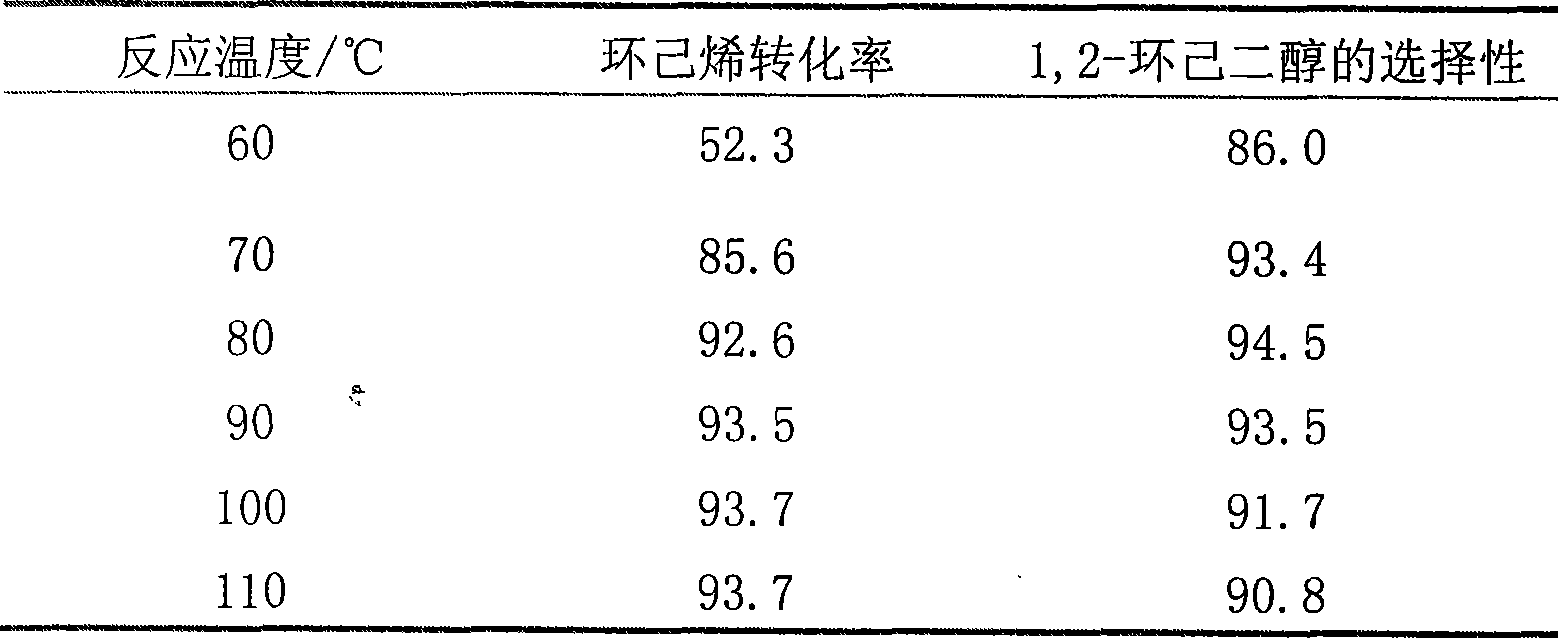
Method for preparing 1,2-cyclohexanediol by catalytic oxidation of cyclohexene
A technology of catalytic oxidation and cyclohexanediol, applied in the fields of oxidation reaction preparation, organic chemistry, etc., can solve the problems affecting the industrial production of products, high price, complicated process, etc., achieve high industrial application value, reduce production cost, and improve process flow simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add respectively 0.10g molybdenum trioxide, 5.0g toluene, 0.063mol (5.166g) cyclohexene and 0.063mol (65%, 8.74g) tert-butyl hydroperoxide in a 50mL round bottom flask, install a condenser at 80 Heat and stir in an oil bath at ℃ for 10 hours, cool to room temperature, analyze the product by gas chromatography, the conversion rate of cyclohexene is 93.0%, the selectivity of 1,2-cyclohexanediol is 93.5%, and the remaining products are cyclohexene ketones and 2-hydroxycyclohexanone.
Embodiment 2
[0017] Add 0.10g vanadium pentoxide, 5.0g toluene, 0.063mol (5.166g) cyclohexene and 0.063mol (65%, 8.74g) tert-butyl hydroperoxide respectively in a 50mL round bottom flask, and install a condenser tube in Heat and stir in an oil bath at 80°C for 10 hours, cool to room temperature, and analyze the product by gas chromatography. The conversion rate of cyclohexene is 73.7%, the selectivity of 1,2-cyclohexanediol is 91.2%, and the remaining products are epoxy Cyclohexane and cyclohexenone.
Embodiment 3
[0019] Add 0.10g niobium pentoxide, 5.0g toluene, 0.063mol (5.166g) cyclohexene and 0.063mol (65%, 8.74g) tert-butyl hydroperoxide respectively in a 50mL round bottom flask, install a condenser tube in Heat and stir in an oil bath at 80°C for 10 hours, cool to room temperature, and analyze the product by gas chromatography. The conversion rate of cyclohexene is 2.7%, the selectivity of 1,2-cyclohexanediol is 31.3%, and the remaining products are epoxy Cyclohexane and cyclohexenone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com