Non-steroidal male hormone receptor regulating agent and medical uses thereof
An androgen receptor, non-steroidal technology, applied in the field of a class of non-steroidal androgen receptor modulators and their medical applications, can solve the problems such as the use has not been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Synthesis of 3-phenyl-3-(4-nitroanilino)-1-(4-chlorophenyl)-1-propanone (MWW6003)
[0062]
[0063] Add 10.60 grams (0.1mol) of benzaldehyde, 13.81 grams (0.1mol) of 4-nitroaniline, and 150ml of absolute ethanol into the reaction flask, stir at room temperature for 10 minutes, then add 15.46 grams (0.1mol) of 4-chloroacetophenone And catalytic amount of concentrated hydrochloric acid, then stirred at room temperature for 20 hours. After the reaction, the reaction solution was cooled overnight, and the precipitated solid was filtered with suction and washed with absolute ethanol. The resulting solid was suspended in 150ml of 95% ethanol, stirred at room temperature for 2 hours, washed with saturated NaHCO 3 Neutralize the solution to alkaline, continue to stir for 1 hour, filter with suction, wash the filter cake with a small amount of absolute ethanol, and recrystallize the crude product from a mixed solvent of ethanol / water (1:1) to obtain 29.09 g of needle crystal...
Embodiment 2
[0066] Synthesis of 3-phenyl-3-(4-nitroanilino)-1-(4-bromophenyl)-1-propanone (MWW6015)
[0067]
[0068] Add 10.60 grams (0.1mol) of benzaldehyde, 13.81 grams (0.1mol) of 4-nitroaniline, and 150ml of absolute ethanol into the reaction flask, stir at room temperature for 10 minutes, then add 19.91 grams (0.1mol) of 4-bromoacetophenone And catalytic amount of concentrated hydrochloric acid, then stirred at room temperature for 20 hours. After the reaction, the reaction solution was cooled overnight, and the precipitated solid was filtered with suction and washed with absolute ethanol. The resulting solid was suspended in 150ml of 95% ethanol, stirred at room temperature for 2 hours, washed with saturated NaHCO 3 Neutralize the solution to alkaline, continue to stir for 1 hour, filter with suction, wash the filter cake with a small amount of absolute ethanol, and recrystallize the crude product from a mixed solvent of ethanol / water (1:1) to obtain 30.66 g of needle crystals,...
Embodiment 3
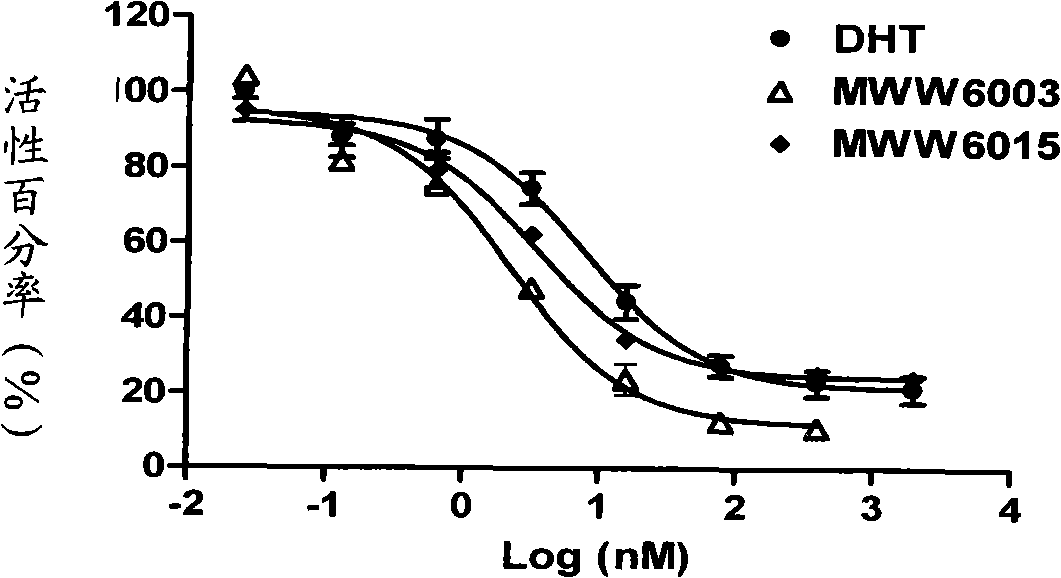
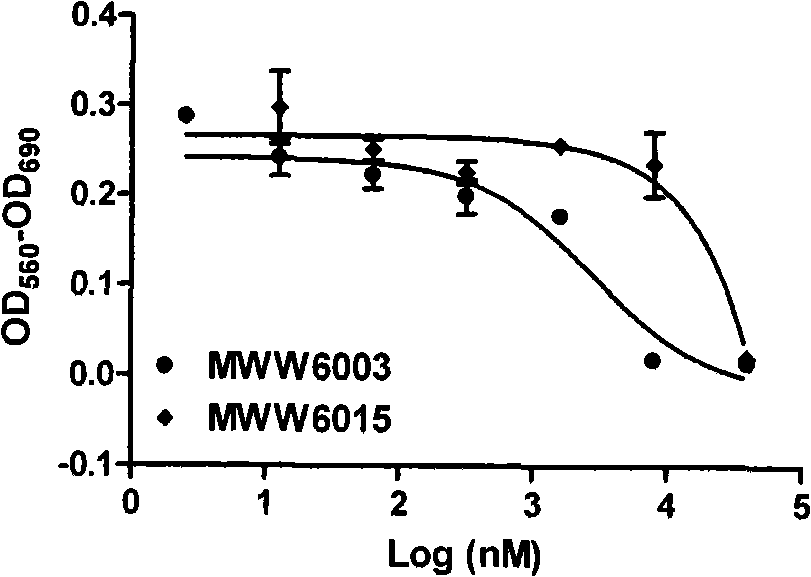
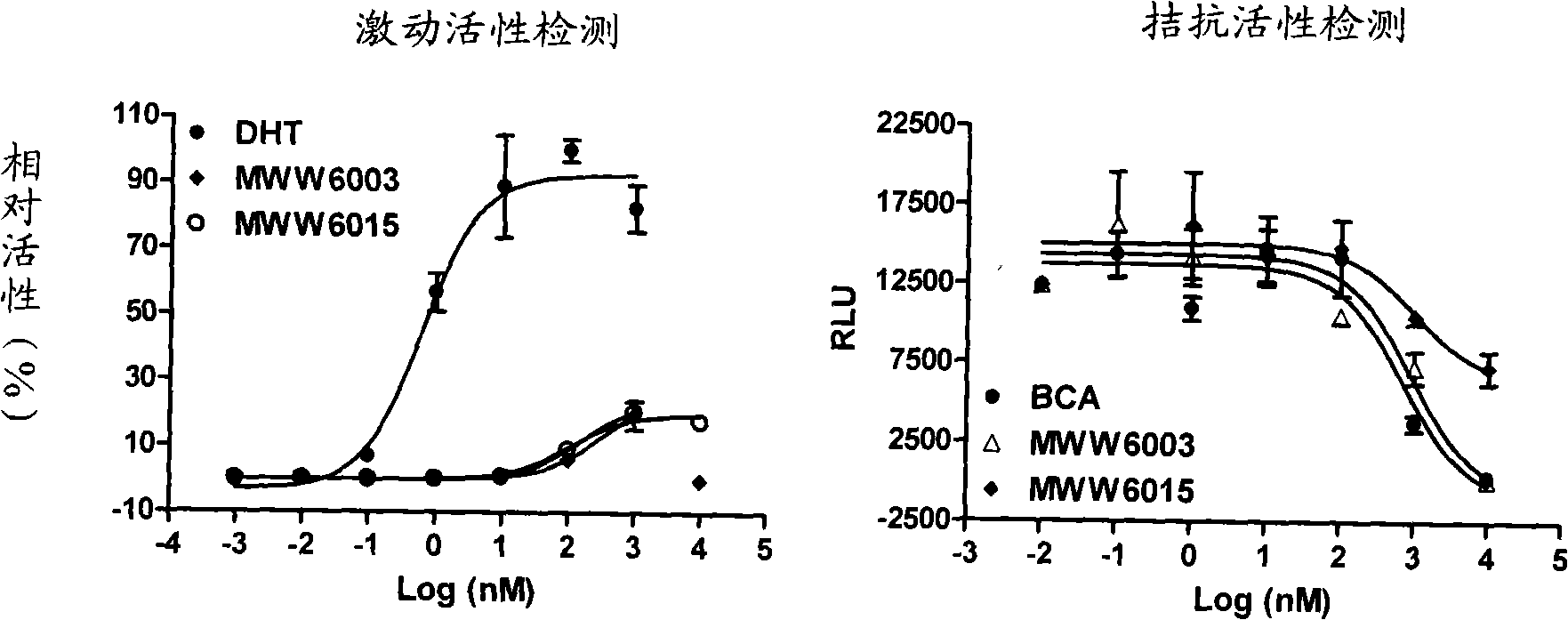
[0071] Biological activity test
[0072] 1. Material equipment
[0073] 1.1 Plasmids and cell lines: androgen receptor expression plasmids and luciferase reporter gene plasmids were constructed by the National Center for Drug Screening; human cervical cancer epithelial cell line HeLa, human breast cancer cell line MDA-MB-453 and human prostate cancer cell line LNCaP were purchased from ATCC, USA.
[0074] 1.2 Reagents: fetal bovine serum (Fetal bovine serum, FBS, GIBCO / BRL, USA); activated carbon and dextran treated fetal bovine serum (CD-FBS, Hyclone, USA); DMEM and RPMI1640 medium (GIBCO / BRL, USA ); IMEM medium (Bioresource, USA), luciferase detection kit (Promega Corporation, USA); Fugene 6 (Roche Ltd., USA); [ 3 H] Dihydrotestosterone (Dehydrotestosterone, DHT, Amersham, UK); scintillation fluid (SuperMixTM, PerkinElmer, USA); androgen receptor protein is the expression product of the receptor gene in insect cells.
[0075] 1.3 Instruments: Envision 2101 Multilabel Read...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com