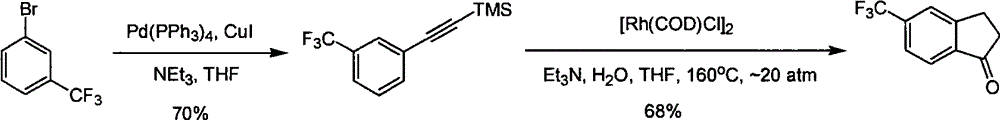
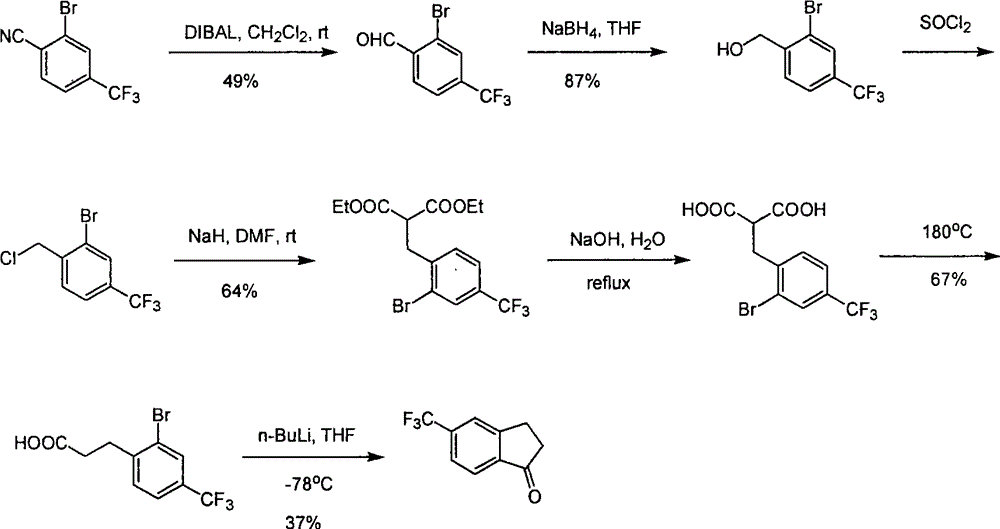
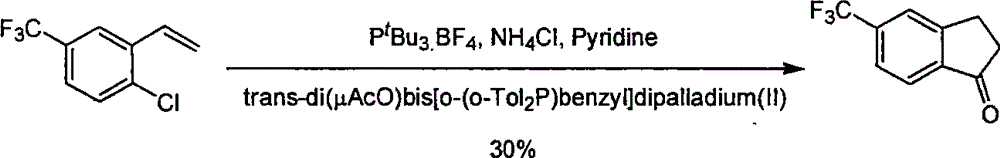Process for synthesizing 5-trifluoromethyl-1-indene ketone
A technology of trifluoromethyl and synthetic methods, applied in the field of new 5-trifluoromethyl-1-indanone synthesis, can solve the problems of harsh reaction conditions, long synthetic routes, expensive catalysts or raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Synthesis of m-trifluoromethylcinnamic acid
[0023]
[0024] Add m-trifluoromethylbenzaldehyde (50 grams, 0.287mol), malonic acid (44.6 grams, 0.429mol), 15.4 milliliters of pyridine, and 0.72 milliliters of piperidine successively in a 500 milliliter three-neck flask equipped with a stirring bar, and start Stir and heat to 100°C for reflux reaction for 3-4 hours. Stop stirring, slowly pour into 300 ml of ice water while hot, slowly add 10% hydrochloric acid therein until the solid is completely precipitated, filter with suction, wash the solid with water, and dry in vacuo to obtain 60 g of a white solid product with a yield of 96.7%. It was directly used in the next reaction without purification. 2. Synthesis of m-trifluoromethylphenylpropionic acid
[0025]
[0026] Dissolve m-trifluoromethylcinnamic acid (58.6 grams, 0.27mol) in 600 milliliters of ethanol, add 10 grams of palladium hydroxide carbon (water content 50%), hydrogenate at room temperature (40 ...
Embodiment 2
[0031] Synthesis of 5-trifluoromethyl-1-indanone
[0032]
[0033] Add 48 ml of trifluoromethanesulfonic acid to a 250 ml three-necked flask, cool to -20°C, add m-trifluoromethylphenylpropionic acid (10 g, 45.87 mmol) dropwise under stirring, and react for 30 minutes and then naturally rise to room temperature , and stirred overnight at room temperature. Pour into ice water, extract three times with dichloromethane, wash with water, dry and concentrate under reduced pressure. Silica gel column chromatography separated 2.0 g of the product with a yield of 21.8%.
Embodiment 3
[0035] Synthesis of 5-trifluoromethyl-1-indanone
[0036]
[0037] Add 420 ml of trifluoromethanesulfonic acid into a 1000 ml three-necked flask, cool to -20°C, add m-trifluoromethylphenylpropionic acid (122 g, 0.56 mol) dropwise under stirring, and naturally rise to -10 mol after 30 minutes of reaction. ℃, stirred overnight at this temperature, poured into ice water, extracted three times with dichloromethane, washed with water, dried and concentrated under reduced pressure.
[0038] Silica gel column chromatography separated 16.7 g of the product with a yield of 14.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com