Biaryl substituted heterocycle inhibitors of lTA4H for treating inflammation
A substituent, phenyl technology, applied in the field of diaryl-substituted heterocyclic inhibitors, can solve the problems of increasing the risk of MI and stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212]
[0213] step 1
[0214] (R)-2-(Toluene-4-sulfonyloxymethyl)-pyrrolidine-1-carboxylate tert-butyl ester: Add (R)-Boc-prolinol (500mg, 2.48mmol ) in pyridine (1.5 mL) was added tosyl chloride (565 mg, 2.96 mmol) in pyridine (1 mL), and the resulting mixture was stirred at 0° C. for 20 minutes, then warmed to room temperature. The mixture was stirred at room temperature for 8 hours. The solvent was removed from the resulting suspension, and 1N aqueous HCl was added to the crude product, extracted with EtOAc. The organic layer was saturated NaHCO 3 Wash with aqueous solution, then water and brine. Anhydrous Na for organic layer 2 SO 4 Drying and removal of solvent in vacuo afforded the title product (800 mg, 91%) as a viscous oil: MS; m / z 378 (M+Na); 1 H NMR (400MHz, CDCl 3 ); 4.07-4.14 (m, 2H), 7.34 (br s, 2H), 7.77 (d, 2H, J = 8.0 Hz); HPLC (ELSD); 99%.
[0215] step 2
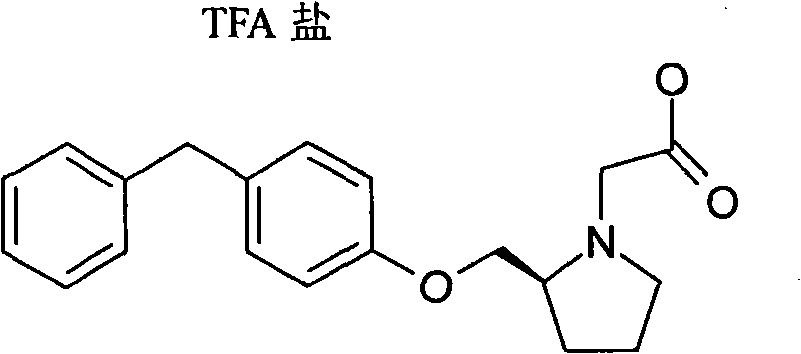
[0216] (R)-2-(4-Benzyl-phenoxymethyl)-pyrrolidine-1-carboxylic acid tert-butyl ester: A...
Embodiment 2
[0220]
[0221] step 1
[0222] (S)-2-(Toluene-4-sulfonyloxymethyl)-pyrrolidine-1-carboxylic acid tert-butyl ester: at 0°C, to S-(-)-1-Boc-2-pyrrolidine To a solution of methanol (22 g, 110 mmol) in pyridine (56 mL) was added a solution of p-toluenesulfonyl chloride (22.9 g, 120 mmol) in pyridine (56 mL) portionwise over 5 minutes. The resulting pale yellow reaction mixture was stirred at 0 °C for 2 hours, then at room temperature overnight. Pyridine was removed in vacuo. The crude oil was extracted into ethyl acetate (400 mL), washed sequentially with 0.5M HCl (100 mL), saturated NaHCO 3 Aqueous solution (100 mL) and brine (100 mL) washed. The combined organic layers were washed with anhydrous Na 2 SO 4 Drying, filtration and concentration in vacuo afforded the title compound (39 g, >100%) as a yellow oil;
[0223] step 2
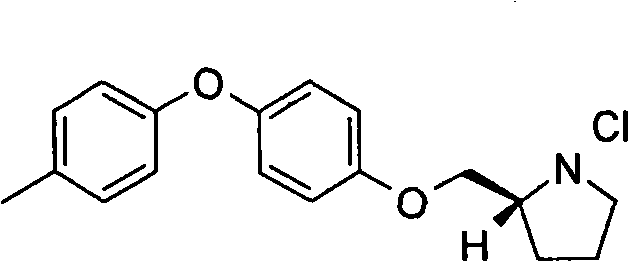
[0224] (S)-2-(4-Benzyl-phenoxymethyl)-pyrrolidine-1-carboxylic acid tert-butyl ester: Add 4-hydroxydiphenylmethane (0.77g, 4.18mmol) at 0°C To...
Embodiment 3
[0228]
[0229] step 1
[0230] 4-[(S)-2-(4-Benzyl-phenoxymethyl)-pyrrolidin-1-yl]-butyric acid methyl ester: To Example 2 (1.5 g, 4.94 mmol) in DMF (23 mL) Potassium carbonate (1.4 g, 10.1 mmol) and methyl 4-bromobutyrate (0.72 mL, 6.26 mmol) were added to the solution in . The resulting slurry was stirred overnight at room temperature. The solvent was concentrated under reduced pressure and the crude product was taken up in ethyl acetate. The organic layer was washed with water, brine, and Na 2 SO 4 Dry, filter and concentrate in vacuo. The crude product was purified by flash chromatography on silica gel using hexane / EtOAc (gradient system) to afford the title compound (0.73 g, 40%) as a yellow oil.
[0231] step 2
[0232] 4-[(S)-2-(4-Benzyl-phenoxymethyl)-pyrrolidin-1-yl]-butyric acid: To a solution of the product from Step 1 (0.13 g, 0.35 mmol) was added 2N NaOH (0.29mL, 0.58mmol) and 80% MeOH / H 2 O (4 mL). The resulting slurry was stirred at 50°C for 67 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com