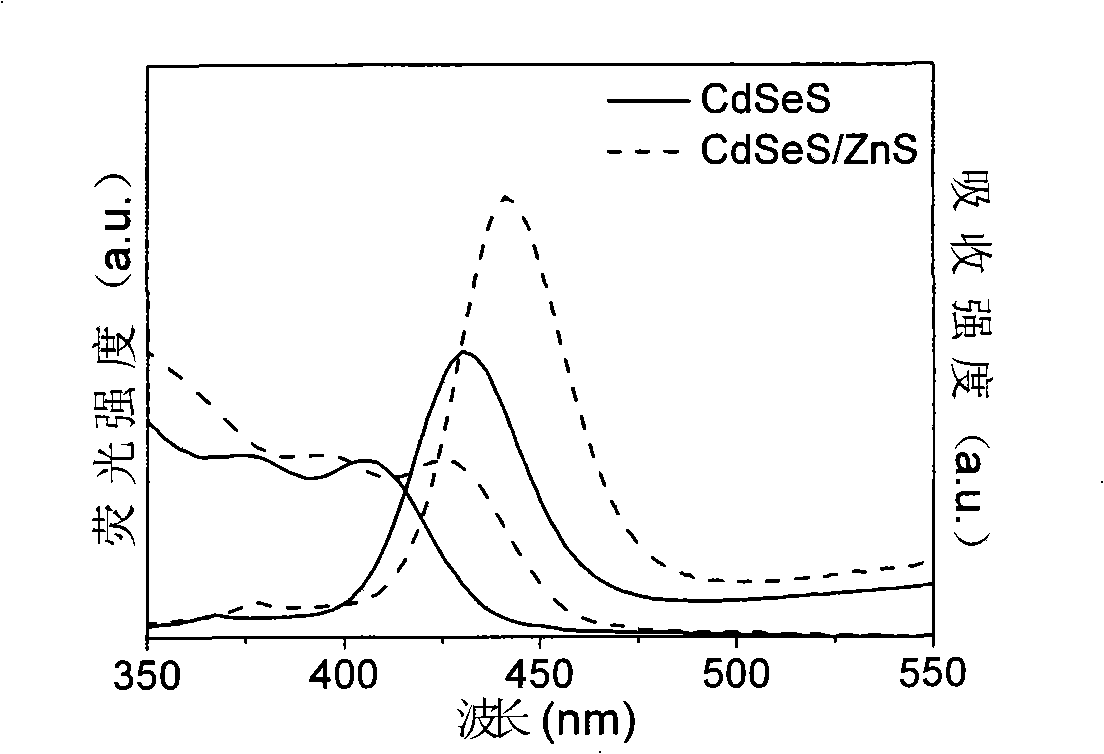
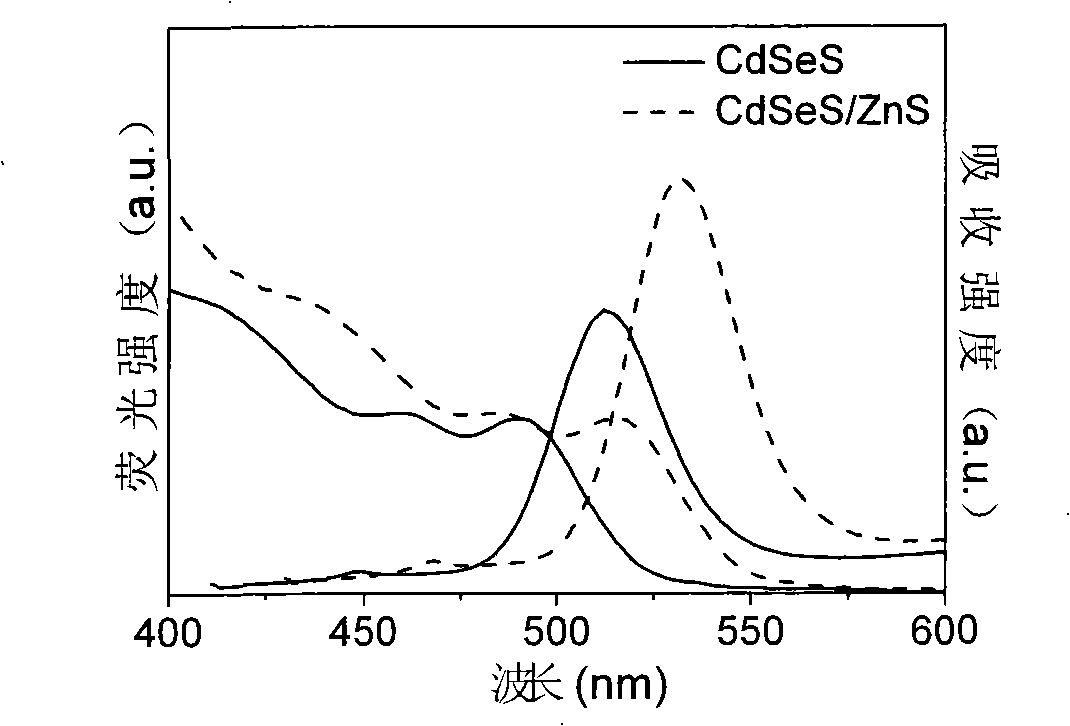
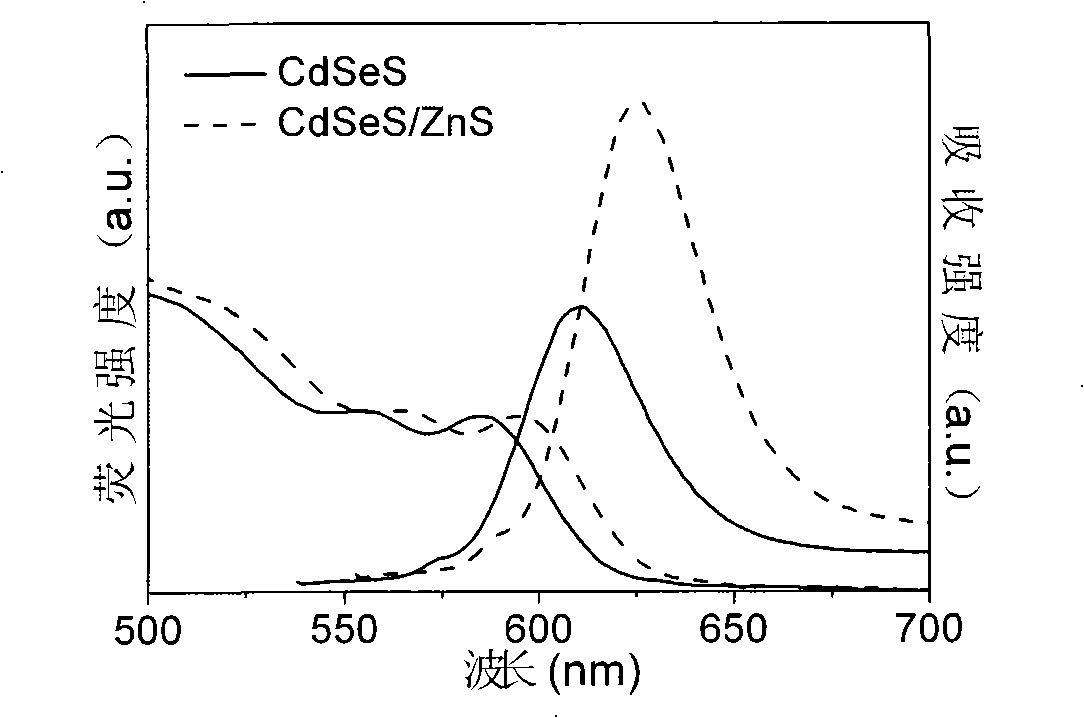
Production method for CdSeS and CdSeS/ZnS core-shell type quantum point
A quantum dot, core-shell type technology, applied in the field of preparation of CdSeS and CdSeS/ZnS core-shell type quantum dots, can solve the problems of poor fluorescence performance, limited scale preparation, high synthesis temperature, etc., and achieve strong fluorescence performance and stability , Low preparation cost and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (a) Preparation of Cd precursor solution. Measure 0.32mL of oleic acid and 9.68mL of liquid paraffin, mix them in a three-neck bottle A, heat to 150°C, add 0.1284g of CdO powder, so that the molar concentration ratio of Cd to oleic acid is 1:1, and wait until the CdO powder is completely dissolved Afterwards, obtain the Cd precursor solution that concentration is 0.1mol / L;
[0030] (b) Preparation of mixed solution of Se and S precursors. Measure 10mL of liquid paraffin into three-neck bottle B, add 0.002g of Se powder, stir and heat the solution to 220°C to completely dissolve the Se powder to obtain Se precursor solution; measure 10mL of liquid paraffin into three-neck bottle C 0.0056g of S powder was added, and the solution was stirred and heated to 120°C to completely dissolve the S powder to obtain the S precursor solution; after mixing the two, a mixed solution of Se and S precursors with a total molar concentration of 0.01mol / L was obtained, wherein The molar c...
Embodiment 2
[0037] (a) Preparation of Cd precursor solution. Measure 1.7068g of stearic acid and 10mL of liquid paraffin, mix them in a three-neck bottle A, heat to 150°C, add 0.2665g of cadmium acetate powder, so that the molar concentration ratio of Cd to stearic acid is 1:3, and the acetic acid After the cadmium powder was completely dissolved, a Cd precursor solution with a concentration of 0.2 mol / L was obtained.
[0038] (b) Preparation of mixed solution of Se and S precursors. Measure 10mL of liquid paraffin into three-neck bottle B, add 0.0158g of Se powder, stir and heat the solution to 220°C to completely dissolve the Se powder to obtain Se precursor solution; measure 10mL of liquid paraffin into three-neck bottle C , add 0.0064g S powder, stir and heat the solution to 120°C to completely dissolve the S powder to obtain the S precursor solution; after mixing the two, a mixed solution of Se and S precursors with a total molar concentration of 0.02mol / L is obtained, wherein The ...
Embodiment 3
[0044] (a) Preparation of Cd precursor solution. Weigh 5.13g of palmitic acid and 6mL of liquid paraffin and mix them in a three-neck bottle A, heat to 150°C, add 1.066g of cadmium stearate powder, so that the molar concentration ratio of Cd to palmitic acid is 1:5, After the cadmium stearate powder was completely dissolved, a Cd precursor solution with a concentration of 0.4 mol / L was obtained.
[0045] (b) Preparation of mixed solution of Se and S precursors. Measure 10mL of liquid paraffin into three-neck bottle B, add 0.0553g of Se powder, stir and heat the solution to 240°C to completely dissolve the Se powder to obtain Se precursor solution; measure 10mL of liquid paraffin into three-neck bottle C , add 0.0032g S powder, stir and heat the solution to 120°C to completely dissolve the S powder to obtain the S precursor solution; after mixing the two, a mixed solution of Se and S precursor with a total molar concentration of 0.04mol / L is obtained, wherein Se The ratio of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com