Preparation method of porous copper powder
A technology of porous copper and copper powder, applied in the field of powder metallurgy, can solve the problems of complex equipment, high powder activity, high bulk density, and achieve the effect of environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
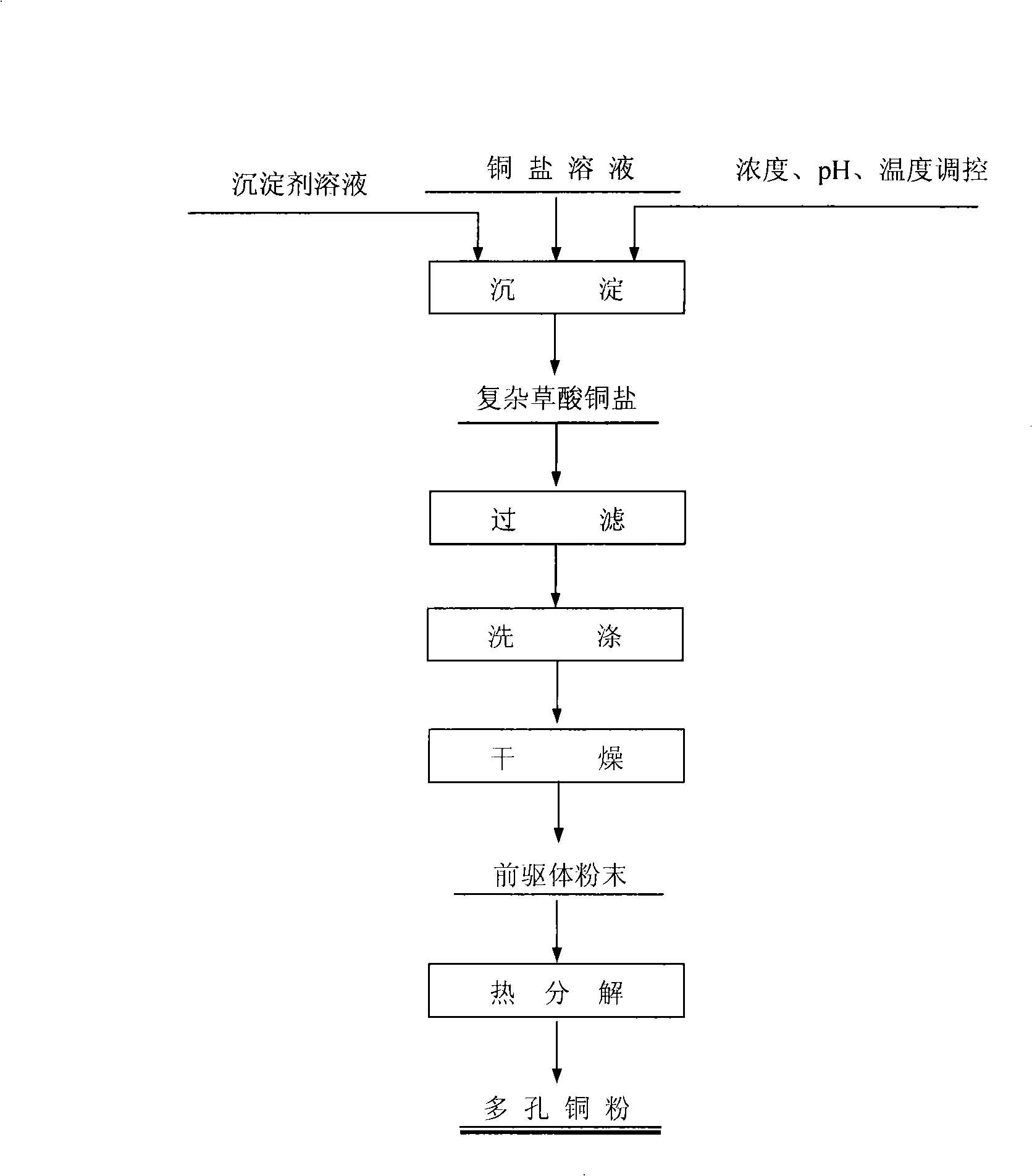
[0016] figure 1 The preparation process of porous copper powder is described:
[0017] ①The soluble copper salt solution and the stoichiometric precipitant solution are added to the reactor through the feeding device in a certain feeding way, and the Cu 2+ -NH 3 -NH 4 + -SG n- -C 2 o 4 2- -H 2 O(SG n- Representative acid ion: SO 4 2- , Cl - , NO 3 - , CH 3 COO - ) system for liquid-phase coordination precipitation, the temperature is controlled at 30-80°C, the pH is adjusted to 3.0-8.5 with ammonia water, and the initial Cu in the feed solution 2+ The concentration is 0.1-1.0mol / L; the dispersant PVP (polyvinylpyrrolidone) is added (the weight percentage of the total solution weight is 0.1%-0.2%);
[0018] ② After the reaction is completed, the precipitate obtained is washed, filtered and dried to become the precursor of copper powder;
[0019] ③Place the precursor of copper powder in an electric furnace regulated by PID, control the temperature at 300-400°C...
Embodiment 1
[0022] The dispersant PVP of copper sulfate solution, oxalic acid solution and 0.1%wt (percentage by weight) is added to the reactor through the feeding device, in Cu 2+ -NH 3 -NH 4 + -SO 4 2- -C 2 o 4 2- -H 2 Coordination precipitation transformation is carried out in the O system, the temperature is controlled at 50 °C, the pH is adjusted to 3.5 with ammonia water, and the initial Cu in the feed solution 2+ Concentration is 0.1mol / L, C 2 o 4 2- The concentration is 0.11mol / L.
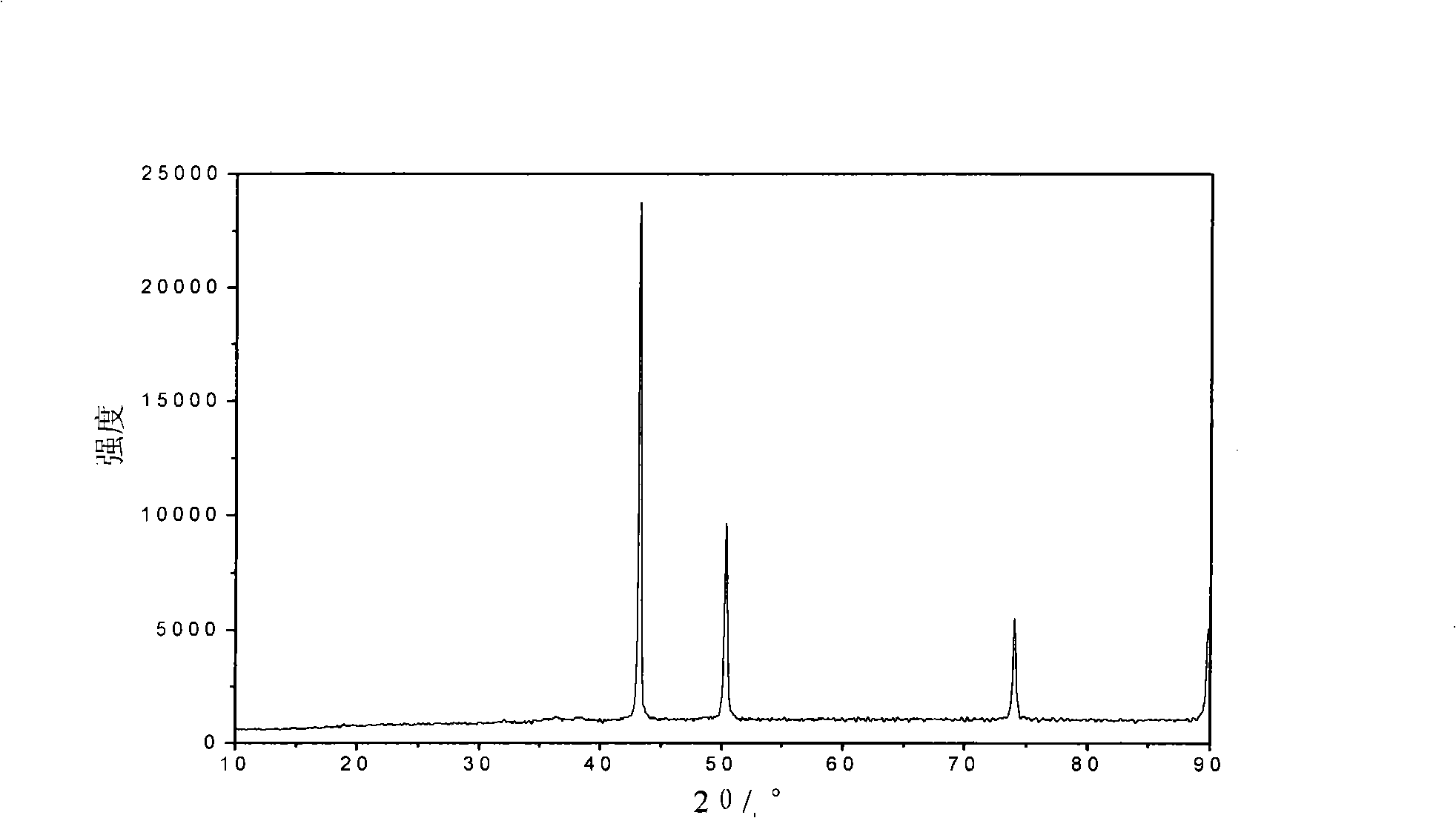
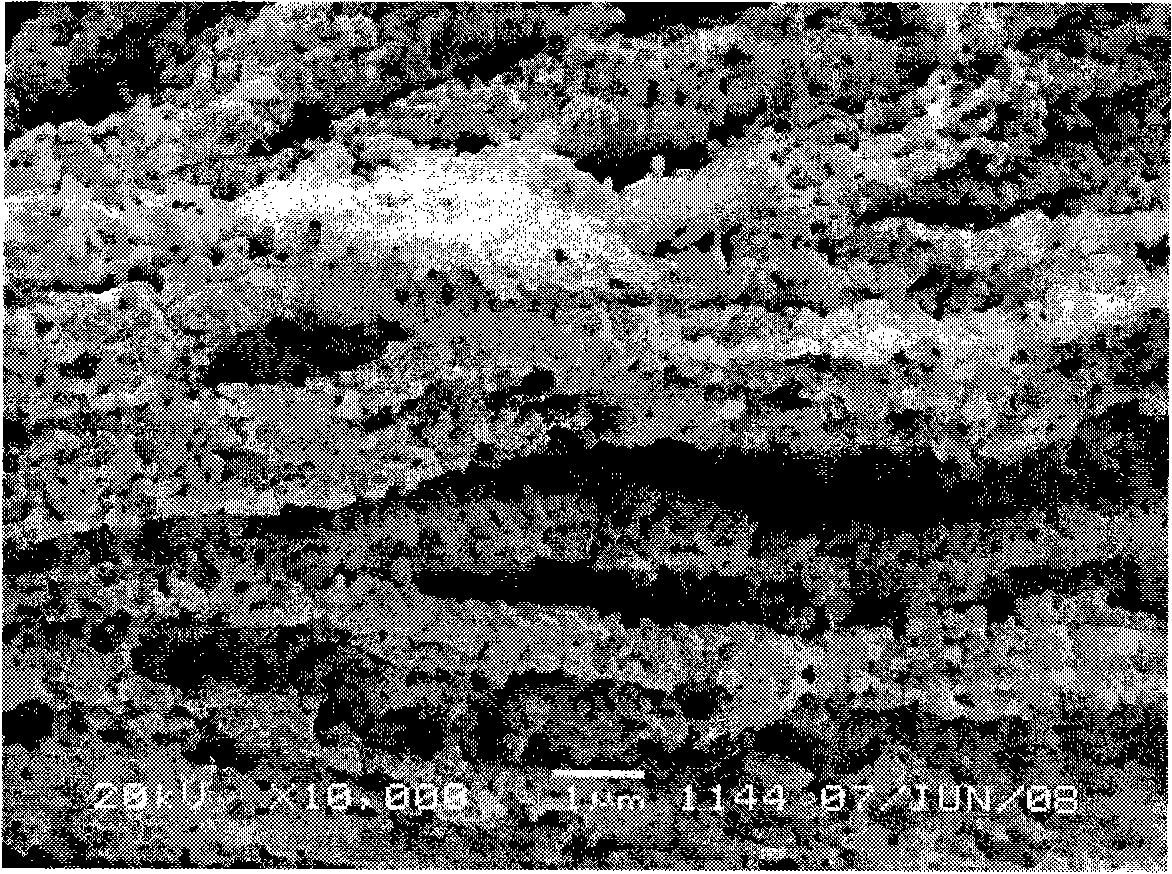
[0023] After the reaction is completed, the obtained precipitate is washed, filtered and dried to become the copper powder precursor. After that, the precursor is put into a burning boat and placed in a PID-regulated electric furnace. The temperature is controlled at 310 ° C and heated in N 2 The thermal decomposition is carried out under the control of the atmosphere. After the thermal decomposition is completed, cool to room temperature, and the deep red powder taken out from the burnin...
Embodiment 2
[0025] The copper chloride solution, the ammonium oxalate solution and the dispersant PVP of 0.2%wt are added to the reactor through the feeding device, and the Cu 2+ -NH 3 -NH 4 + -Cl - -C 2 o 4 2- -H 2 Coordination precipitation transformation is carried out in the O system, the temperature is controlled at 70 °C, the pH is adjusted to 6.5 with ammonia water, and the initial Cu in the feed solution 2+ Concentration is 0.4mol / L, C 2 o 4 2- The concentration is 0.44mol / L.
[0026] After the reaction is completed, the obtained precipitate is washed, filtered and dried to become the copper powder precursor. Afterwards, the precursor is put into a burning boat and placed in a PID-regulated electric furnace. The temperature is controlled at 350 ° C, and in (H 2 +N 2 ) under the control of the atmosphere for thermal decomposition. After the thermal decomposition is completed, cool to room temperature, and the deep red powder taken out from the burning boat after being ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com