Liposomes sodium morrhuate
A technology of sodium morrhuate and body fish, which is applied in the field of medicine, can solve the problems of affecting the normal tissue function around, increasing the pain of children, side effects, etc., and achieves easy mastery and industrial production, good inhibitory therapeutic effect, good stability and The effect of encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Liposomal drug product containing 1% sodium morrhuate
[0079] Liposomal Drug Prescription:
[0080] name
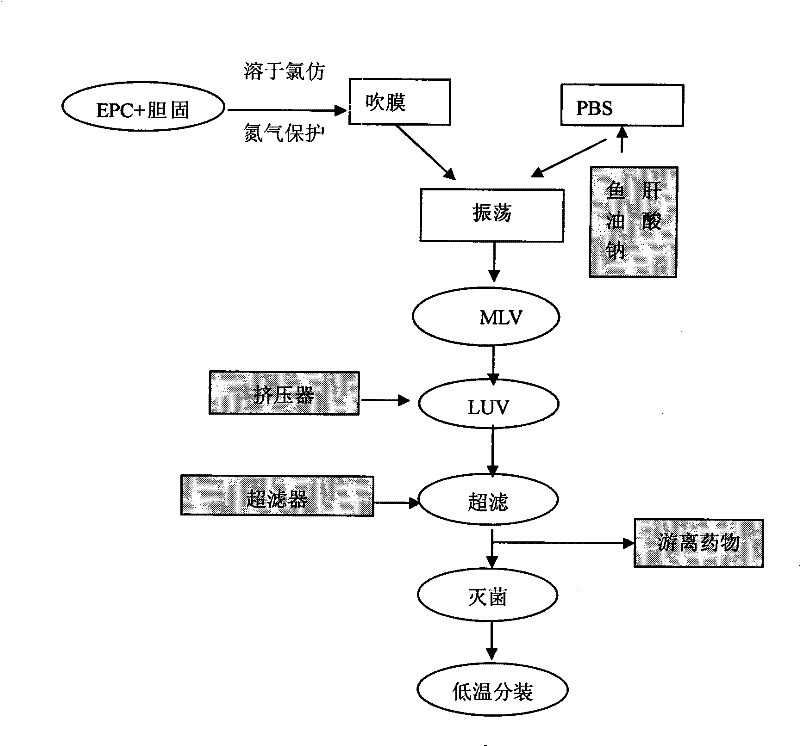
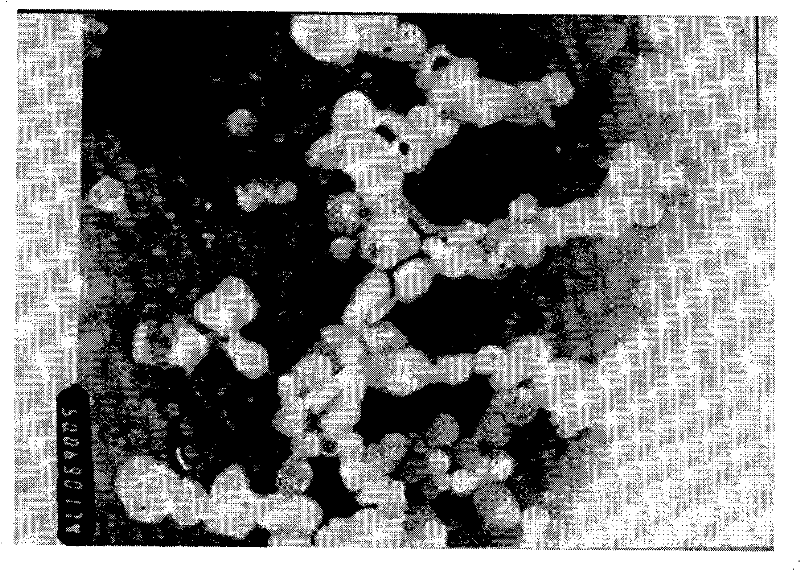
[0081] Weigh EPC and CHO according to the prescription amount and place them in a test tube, add 4ml of chloroform and shake to dissolve them completely. In a water bath at 60°C, blow off the chloroform with nitrogen gas. After all the chloroform is about to be removed, quickly vacuum to form a flocculent film , continue vacuuming for 3h to completely remove chloroform. Weigh 100mg of sodium morrhuate powder and dissolve it in 10ml of PBS (pH6.84), oscillate to dissolve it completely, suck it into a disposable syringe, and slowly drop into the sodium morrhuate solution while oscillating the vacuum-pumped test tube. It must be slow until all the solution is dripped out (the whole process is about 15 minutes), and the test tube forms a yellow-white emulsion and continues to shake for 45 minutes, that is, multi-lamellar liposomes (MLV) are forme...
Embodiment 2
[0082] Example 2: 2.5% sodium morrhuate in liposomal drug product
[0083] Liposomal Drug Prescription:
[0084] name
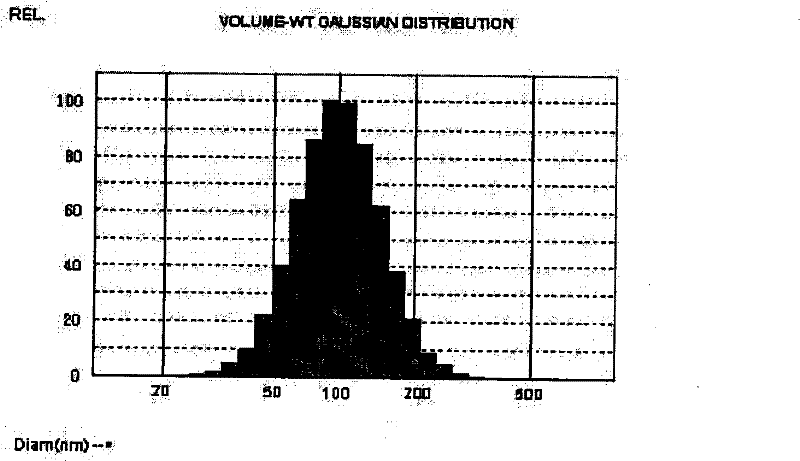
[0085] The above-mentioned raw materials were weighed according to the prescription quantity, and the preparation process was the same as in "Example 1", to obtain liposomal sodium morrhuate with an average particle diameter of 108.4nm ± 39nm.
Embodiment 3
[0086] Example 3: Liposomal drug product containing 5% sodium morrhuate
[0087] Liposomal Drug Prescription:
[0088] name
[0089] The above-mentioned raw materials were weighed according to the prescription quantity, and the preparation process was the same as in "Example 1", that is, liposomal sodium morrhuate with an average particle diameter of 103.6nm ± 32nm was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com