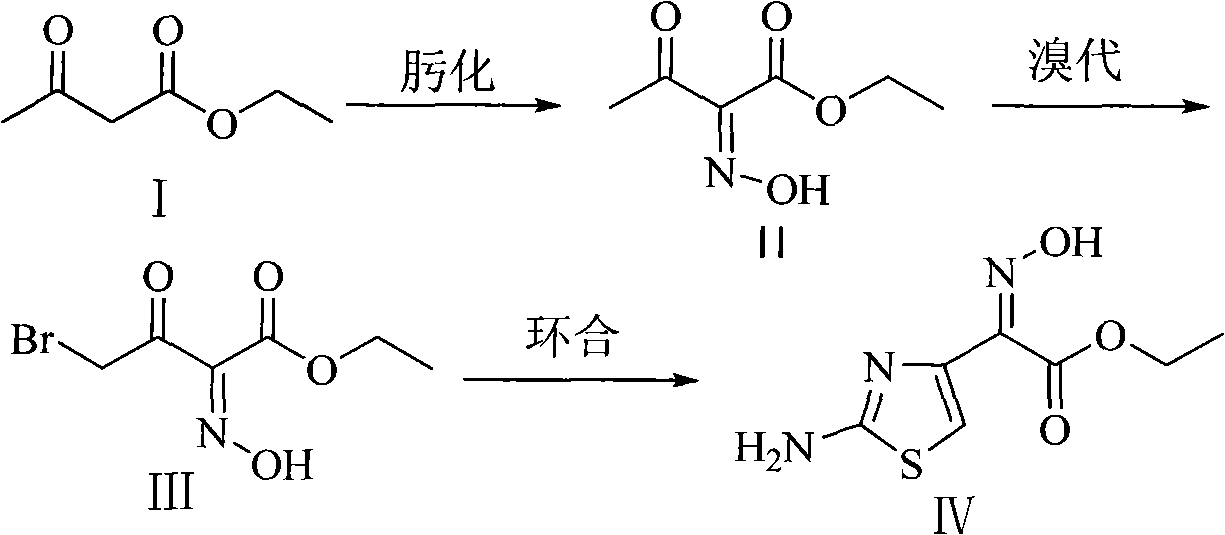
Method for preparing Ethyl 2-(2-aminothiazole-4-yl)-2-hydroxyiminoacetate
The technology of ethyl norfoxamate and ethyl acetoacetate is applied in the field of preparation of ethyl norfoxamate, can solve the problems of long production cycle, troublesome post-processing, many unit operations, etc., and achieves low production cost and simple post-processing. , The effect of simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The ratio of the amount of feed material is ethyl acetoacetate: n-propyl nitrite: bromine: thiourea=1.0: 1.5: 1.3: 2.0. The inorganic base is sodium bicarbonate; the alcoholic solvent is ethanol, and the total consumption is 6 times of the input mass of ethyl acetoacetate.
[0032] (1) In a 3L three-necked flask, add ethyl acetoacetate (208.0g, 1.6mol), ethanol (624.0g), 0°C (t 1 ) was slowly added dropwise n-propyl nitrite (213.6g, 2.4mol), and the oximation reaction time was 8 hours (T 1 ), the resulting reaction solution was directly used for the bromination reaction.
[0033] (2) At 8°C (t 2 ), dropwise the mixed solution of bromine (332.8g, 2.08mol) and ethanol (624.0g), the bromination reaction time was 6 hours (T 2 ), the resulting reaction solution was directly used for the cyclization reaction.
[0034] (3) In a 3L four-necked flask, add an aqueous solution (concentration 20%) of thiourea (243.2g, 3.2mol), and control the temperature at 28°C (t 3 ), dropwi...
Embodiment 2
[0036] The molar ratio of the feed materials is ethyl acetoacetate: n-propyl nitrite: bromine: thiourea=1.0:2.0:1.1:1.5. Inorganic base is sodium carbonate; Alcohol solvent is n-propanol, and total consumption is 6 times of ethyl acetoacetate input quality (in step (1) and step (2), n-propanol consumption is respectively 3 times of ethyl acetoacetate quality ).
[0037] Others are the same as embodiment 1. The yield is 77.1%, the melting point is 185.5-186.1°C, and the HPLC purity is 99.45%.
Embodiment 3
[0039] The molar ratio of the feed materials is ethyl acetoacetate: n-propyl nitrite: bromine: thiourea=1.0:0.8:1.1:4.0. Inorganic base is sodium hydroxide; Alcohol solvent is methyl alcohol, and total consumption is 15 times of ethyl acetoacetate input quality (methanol consumption is 10 times of ethyl acetoacetate in the step (1), and methyl alcohol consumption is in the step (2). 5 times that of ethyl acetoacetate).
[0040] Others are the same as embodiment 1. The yield of demethylaminothiaxamic acid ethyl ester was 72.6%, the melting point was 185.6-186.1°C, and the HPLC purity was 99.51%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com