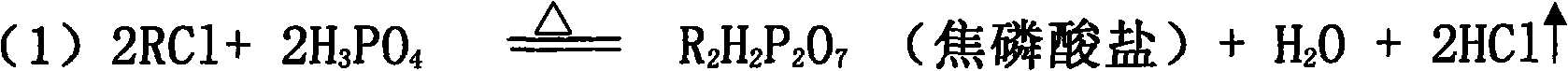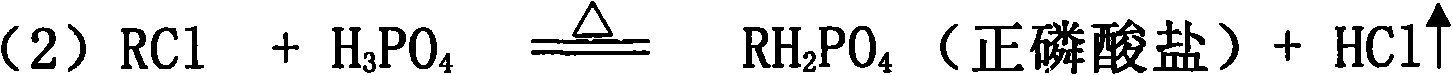Method for preparing dihydric phosphate
A technology of dihydrogen phosphate and phosphoric acid, which is applied in the direction of phosphate, phosphorus oxyacid, etc., can solve the problems of large aspect ratio, difficulty in large-scale production, small effective volume, etc., and achieve the effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
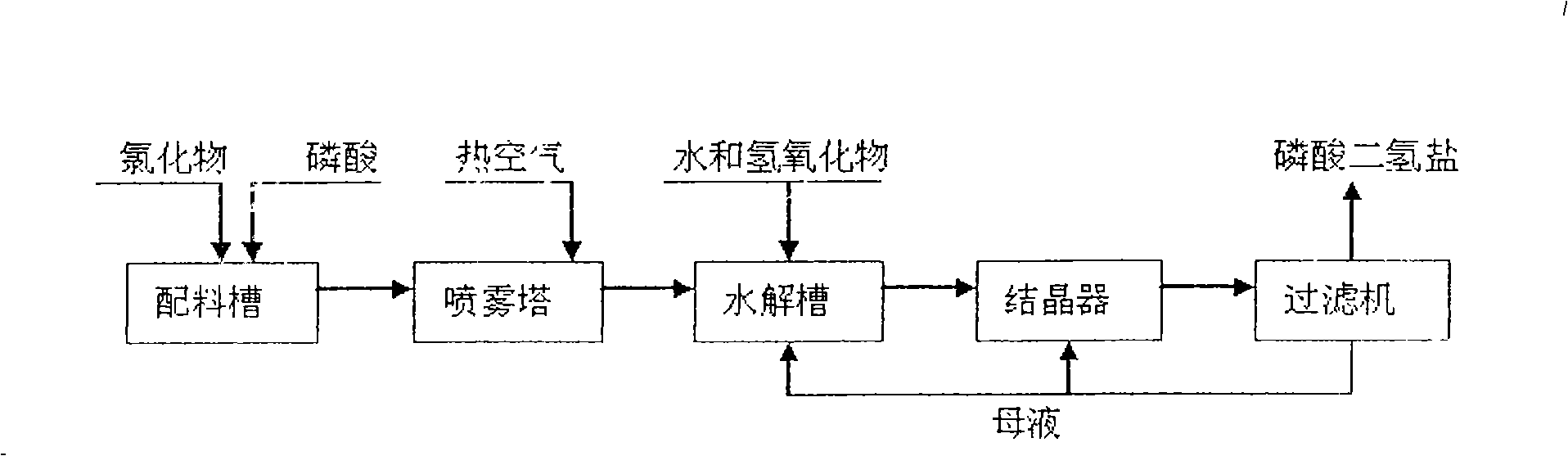
[0029] will contain K 2 O 59.7%; Cl - 46.73% KCl was added to the P 2 o 5 24.88% phosphoric acid solution, stir evenly in the batching tank, adjust H 3 PO 4 The molar ratio of / KCl was 1.3. Transport the feed liquid to the spray drying tower for atomization, use high-temperature air for heating, control the feed volume of the atomizer and the temperature of the inlet air, and detect that the inlet temperature of the atomization drying tower is 700°C, and the outlet temperature of the tower is 400°C. At this temperature, potassium chloride and phosphoric acid complete the double decomposition reaction, and the bottom material flows into the hydrolysis tank, and potassium hydroxide aqueous solution is added to the hydrolysis tank, and the temperature of the hydrolysis tank is detected to be 108°C, so that potassium pyrophosphate is hydrolyzed into potassium dihydrogen phosphate, and the hydrogen oxidation is controlled. The amount of potassium added is such that the pH of t...
Embodiment 2
[0033] A 35% potassium chloride solution was added to the P 2 o 5 40.3% phosphoric acid solution, stir evenly in the batching tank, adjust H 3 PO 4 The / KCI molar ratio is 2.0. Transport the feed liquid to the spray drying tower for atomization, use high-temperature air for heating, control the feed volume of the atomizer and the temperature of the inlet air, and detect that the inlet temperature of the atomization drying tower is 450°C, and the outlet temperature of the tower is 298°C. Potassium chloride and phosphoric acid complete the metathesis reaction at this temperature, and the bottom material flows into the hydrolysis tank, and potassium carbonate aqueous solution is added to the hydrolysis tank, and the temperature of the hydrolysis tank is detected to be 120°C, so that the generated potassium pyrophosphate is hydrolyzed into potassium dihydrogen phosphate, and controlled The amount of potassium carbonate added is such that the pH of the hydrolyzate is 4.4-4.6, th...
Embodiment 3
[0037] Add industrial sodium chloride with a content of 98.9% to the 2 o 5 23.98% phosphoric acid solution, stir evenly in the batching tank, adjust H 3 PO 4 The / NaCI molar ratio was 1.05. Transport the feed liquid to the spray drying tower for atomization, use high-temperature air for heating, control the feed volume of the atomizer and the temperature of the inlet air, and detect that the inlet temperature of the atomization drying tower is 430°C, and the outlet temperature of the tower is 250°C. At this temperature, sodium chloride and phosphoric acid complete the metathesis reaction, the bottom material flows into the hydrolysis tank, and sodium hydroxide aqueous solution is added to the hydrolysis tank, and the temperature of the hydrolysis tank is detected to be 96°C, so that the generated sodium pyrophosphate is hydrolyzed into sodium dihydrogen phosphate, Control the amount of sodium hydroxide added so that the pH of the hydrolyzate is 4.1-4.7, the hydrolyzate that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com