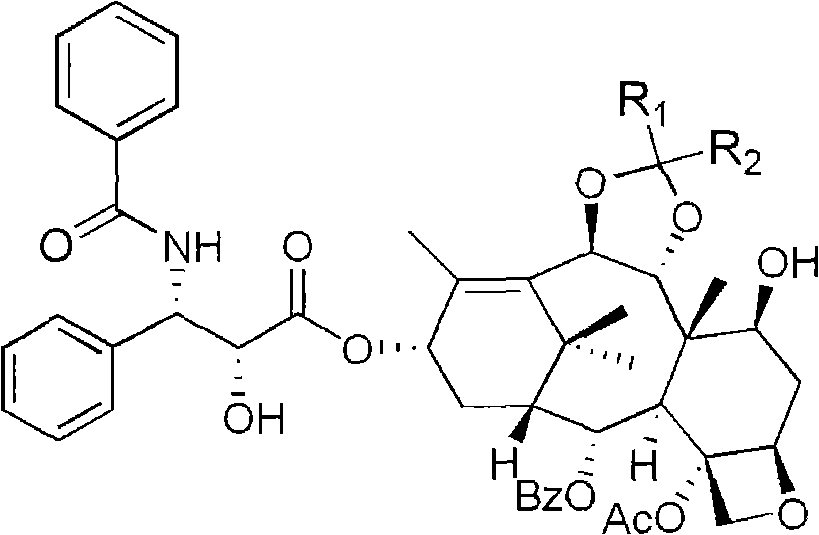
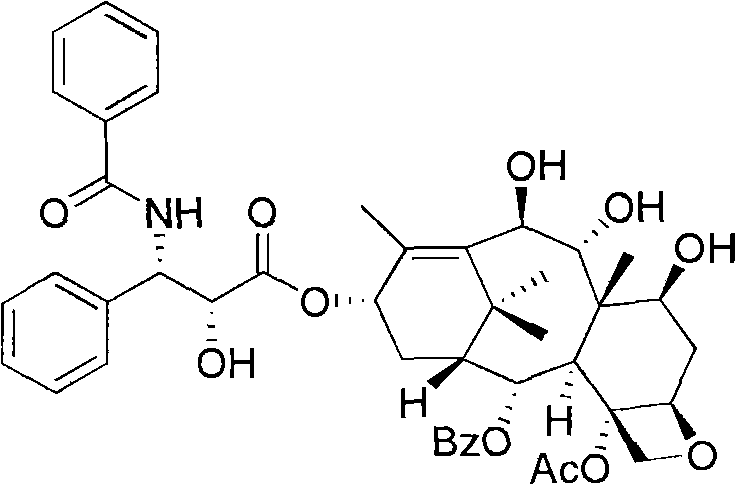
10- deacetylate-9(R)-hydrogenization-1-deoxypaclitaxel analogue and preparation thereof
A paclitaxel analogue and deacetylation technology, which is applied in organic chemistry, drug combination, pharmaceutical formula, etc., can solve the problems of limited collection, low water solubility, drug resistance and side effects, and achieve simple operation, high yield, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: The specific synthesis steps of 10-deacetyl-9(R)-hydrogenated-9,10-O-isopropylidene-1-deoxypaclitaxel
[0030]
[0031] a. 1-Deoxybaccatin VI1 (419 mg, 0.6 mmol) was dissolved in 20 mL of 95% ethanol, 10 mL of hydrazine hydrate was added, and stirred at room temperature for 15 hours. Neutralize with 0.2N hydrochloric acid, extract with ethyl acetate, wash the organic layer three times with water, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure. The crude product was recrystallized with a mixed solvent of methanol and n-hexane to obtain 2277 mg of colorless transparent crystal 7,9,10,13-tetradeacetyl-1-dehydroxybaccatin VI with a yield of 87%;
[0032] b. Compound 2 (239 mg, 0.5 mmol) was dissolved in 18 mL of anhydrous CH 2 Cl 2 and 1.5 mL anhydrous CH 3 OH, after completely dissolving, add 2,2-dimethoxypropane (0.4mL, 2.0mmol), stir evenly, add Mont K10 to make 24mg, stir at room temperature for 0.5 hour; After the ...
Embodiment 2
[0035] Example 2: The specific synthesis steps of 10-deacetyl-9(R)-hydrogenated-1-deoxypaclitaxel
[0036]
[0037] e. Compound 6 (42 mg, 0.05 mmol) was dissolved in 4 mL of methanol, stirred evenly, and 0.1 mL of 0.1 N HCl solution was added at room temperature, and a large amount of white precipitates appeared. Heat the oil bath to 65°C, transfer to room temperature after 6 hours, add saturated NaHCO 3 The solution was neutralized; extracted with ethyl acetate, the organic layer was washed three times with water, and anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and the initial product was slowly separated by column chromatography (thin-layer chromatography silica gel, petroleum ether: ethyl acetate = 1:2) to obtain 10-deacetyl-9(R)-hydrogenated-1 - deoxypaclitaxel 34mg, yield rate 86%; 1 H NMR (500Hz, CDCl 3 ): δ1.13(s, 3H, CH 3 ), 1.53 (s, 3H, CH 3 ), 1.55-1.57 (m, 1H), 1.73 (s, 3H, CH 3 ), 1.78 (s, 3H, CH 3 ), 1.88-1.94(m, 2H)...
Embodiment 3
[0038] Example 3: In vitro screening experiment for anti-tumor biological activity
[0039] MTT method: 3-5×10 4 cells / mL cell suspension 100 μL, set at 37°C, 5% CO 2 Inside the incubator. After 24 hours, add sample solution, 10 μL wells, set up double wells, 37 ° C, 5% CO 2 The effect is 72h. Add 20 μL of 5 mg / mL MTT solution to each well, and add the dissolving solution after 4 hours of action, 100 μL / well, put it in an incubator, measure the 570nm OD value with an MK-2 automatic microplate reader after dissolution.
[0040] Table 1 Inhibitory effect of compound 20 and taxol on human tumor cell proliferation in vitro
[0041]
[0042] A549 (human lung cancer cells); A2780 (human ovarian cancer cells).
[0043] It can be seen from Table 1 that compounds 6 and 7 also have inhibitory effects on the growth of human lung cancer cell A549 and human ovarian cancer cell A2780.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com