Preparation of progestational hormone
A technology of dienogest and its compound, which is applied in the field of preparation of dienogest, can solve the problems of compound 1 difficult to obtain, harsh reaction conditions, difficult industrialization, etc., and achieve the effect of good effect, mild conditions, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
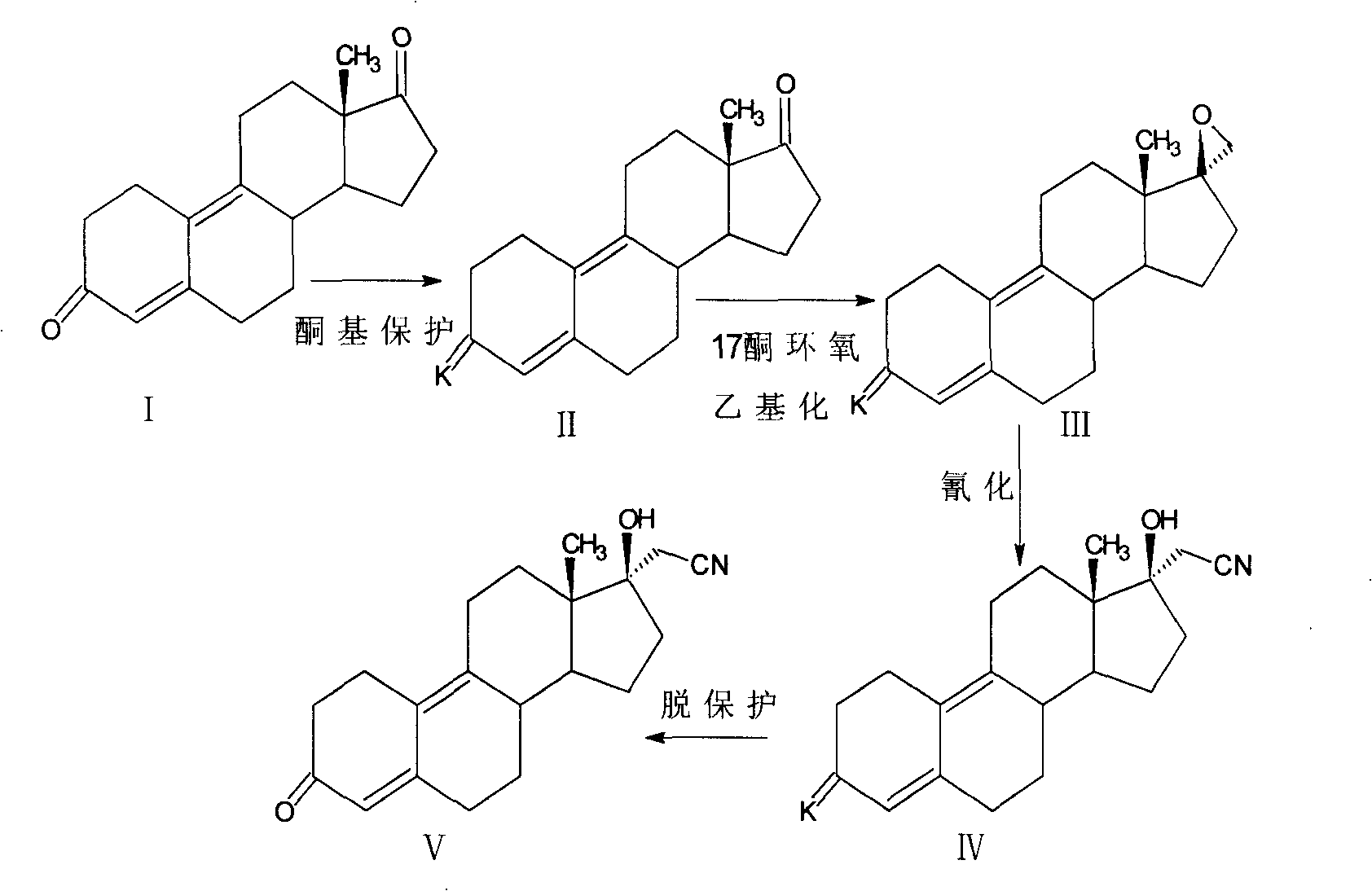
[0070] The synthesis of embodiment one dienogest
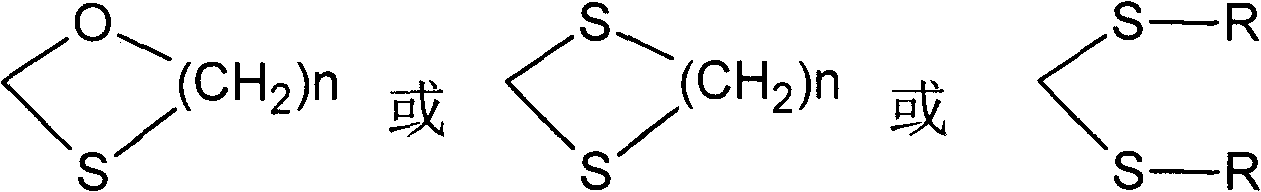
[0071] (1) Estro-4,9(10)-diene-3,17-dione-3,3-ethylidene ketone thiol (formula VI):
[0072] Add 10g of estro-4,9-diene-3,17-dione (Pharmaceutical Industry, 1985, 16(9):15-18) and 100ml of methanol into the reaction flask, blow nitrogen, stir, add 4ml of hydroxyethylene dione Mercaptan and 5ml boron trifluoride etherate, react at 25±2°C for 2h, distill off methanol, dissolve the residue in dichloromethane, wash with saturated aqueous sodium bicarbonate, wash with water, concentrate the separated organic layer, and Crystallization in cyclohexane gave the expected product 11.9 g estra-4,9(10)-diene-3,17-dione-3,3-ethylidene ketone mercaptan; IR: 3033 (v=CH ), 2926 (vCH 3 ), 2881 (vCH 2 ), 1735(vC=O), 1638(vC=C), 1062(vC-O-C); H-NMR, 0.88(18-CH 3 ), 3.33 (-S-CH 2 ), 3.99 (-O-CH 2 ), 5.59(4-H);
[0073](2) 17β-spiro-1′, 2′-oxirane-estra-4,9(10)-diene-3-ketone-3,3-ethylidene ketone thiol (formula VII ): under nitrogen, add ...
Embodiment 2
[0081] The synthesis of embodiment two dienogest
[0082] (1) Estra-4,9(10)-diene-3,17-dione-3,3-ethanethiol ketal (formula IX):
[0083] Add 10g of estro-4,9-diene-3,17-dione and 50ml of dichloromethane into the reaction flask, blow nitrogen, stir, and add 10g of NaHSO 4 .SiO 2 (Synthesis 2005, 2, 250-254), add 8ml of ethanethiol, react at 25±2°C for 1h, filter, wash the filtrate with saturated aqueous sodium bicarbonate, wash with water, concentrate the separated organic layer, and Crystallization in the medium obtained the expected product 12.3g estro-4,9(10)-diene-3,17-dione-3,3-ethanethiol ketal;
[0084] IR: 3035 (v=CH), 2928 (vCH 3 ), 2885 (vCH 2 ), 1735 (vC=O), 1639 (vC=C);
[0085] H-NMR, 0.89 (18-CH 3 ), 0.97 (-CH on ethyl sulfide 3 ), 3.30 (-S-CH 2 ), 5.58(4-H);
[0086] (2) 17β-spiro-1′, 2′-oxirane-estro-4,9(10)-dien-3-one-3,3-ethanethiol ketal (Formula X):
[0087] Under nitrogen, add 6g of sodium methoxide to the reaction flask, add 70ml of tetrahydrofu...
Embodiment 3
[0098] The synthesis of embodiment three dienogest
[0099] (1) Estro-4,9(10)-diene-3,17-dione-3,3-ethylene ketone thiol:
[0100] Add 10g of estro-4,9-diene-3,17-dione and 70ml of dioxane into the reaction flask, blow nitrogen, stir, add 4ml of ethanedithiol and 5ml of boron trifluoride etherate, 25±2 React at ℃ for 2 h, distill out dioxane, dissolve the residue in dichloromethane, wash with saturated aqueous sodium bicarbonate solution, wash with water, concentrate the separated organic layer, and crystallize in cyclohexane to obtain 11.5 g of the expected product Estro-4,9(10)-diene-3,17-dione-3,3-ethylidene ketone thioketal;
[0101] IR: 3029 (v=CH), 2926 (vCH 3 ), 2879 (vCH 2 ), 1736 (vC=O), 1635 (vC=C);
[0102] H-NMR, 0.87 (18-CH 3 ), 3.20-3.40 (-S-CH 2 -CH 2 -S-), 5.59(4-H);
[0103] (2) 17β-spiro-1′, 2′-oxirane-estra-4,9(10)-dien-3-one-3,3-ethylidene thioketal:
[0104]Under nitrogen, add 7g of sodium ethoxide to the reaction flask, add 100ml of dimethylforma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com