Erythrocyte membrane antigen magnetic ball kit and applications on blood group antibody detection
A technology of red blood cell membrane and kit, applied in the biological field, can solve the problems of difficult acquisition, short storage period, blank antibody screening, etc., and achieve the effect of simple detection method, favorable preservation, and easy automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] Preparation of Erythrocyte Membrane Antigen Magnetic Beads:
[0027] 1. Preparation of erythrocyte antigens:
[0028] (1) Take fresh blood cells, centrifuge to remove the supernatant, and take the packed blood cells for the next step;
[0029] (2) Add SDS to the packed blood cells, and then place at room temperature;
[0030] (3) centrifuge to remove supernatant, leave sediment and add distilled water to the sediment;
[0031] 2. Preparation of magnetic particles:
[0032] (1) FeCl 2 and FeCl 3 Add to distilled water to dissolve;
[0033] (2) under the condition of vigorous stirring, sodium hydroxide is added to the above-mentioned metal salt solution, and it is hydrolyzed into metal composite oxide, thereby obtaining ferric oxide particle solution;
[0034] 3. Cell membrane antigen-coated magnetic particles
[0035] Add the red blood cell membrane antigen obtained in step 2.1 to the ferric tetroxide particle solution obtained in step 2.2, then add polyethylene g...
Embodiment 1
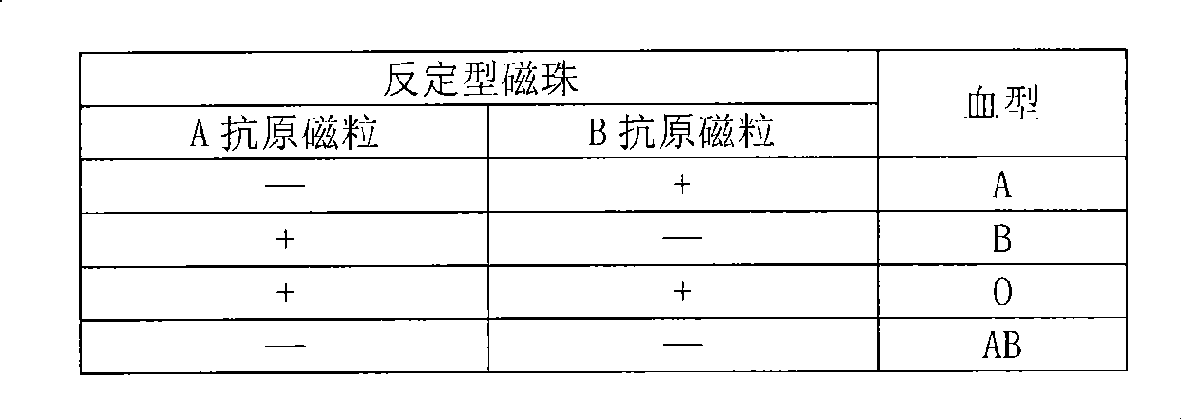
[0041] Example 1 ABO system blood typing test
[0042] A red blood cell membrane antigen magnetic beads and B red blood cell membrane antigen magnetic beads are prepared by using known type A red blood cells and type B red blood cells as in the specific embodiment. Since the red blood cell membrane antigen magnetic beads are coated with A antigen and B antigen, they can replace fresh red blood cells for ABO system blood typing detection. Type A human serum contains anti-B antibodies, type B human serum contains anti-A antibodies, type AB human serum does not contain antibodies, and type O human serum contains anti-AB antibodies. Most of the antibodies of the ABO blood group system are naturally occurring antibodies, which are IgM antibodies. The red blood cell membrane antigen magnetic beads can specifically immunoreact with the corresponding IgM antibodies in the serum to form agglutination visible to the naked eye. The blood type is determined according to the pattern after...
Embodiment 2
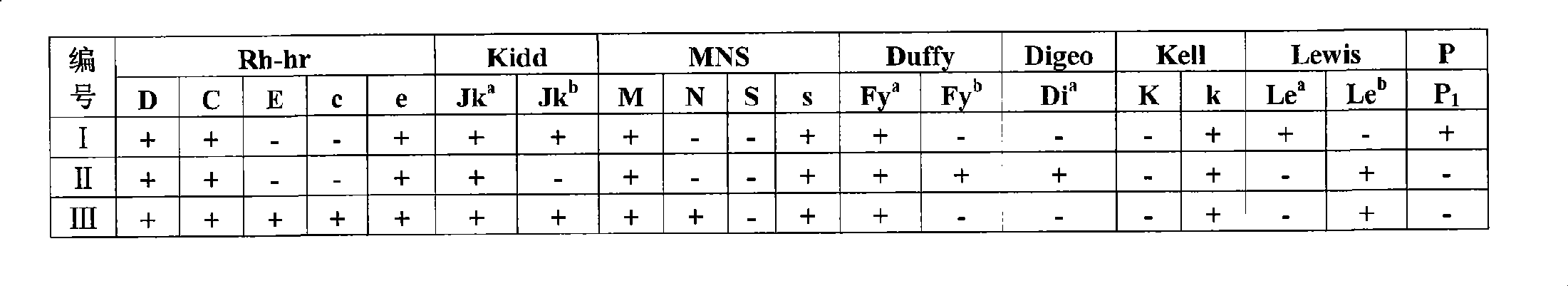
[0046] Example 2 Screening of erythrocyte irregular antibodies
[0047] Red blood cells are detected with antibodies of known specificity, and the antigenic profile of the screened cells is determined as follows:
[0048]
[0049] The above-mentioned screening cells are prepared by screening cell membrane magnetic beads as in a specific embodiment. The surface of the screening cell membrane antigen magnetic beads has all the antigens on the fresh screening cells, which can be used for clinical screening of IgG antibodies. IgG antibodies are generally produced due to alloimmunization such as blood transfusion and pregnancy. It is a 7S globulin with a small molecular weight and a molecular length of only 250A 0, after binding with the corresponding antigen of erythrocytes, it can only bind with erythrocytes in saline medium without visible agglutination reaction, and the sensitized cells must be agglutinated by adding anti-human globulin reagent, so as to detect such antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com