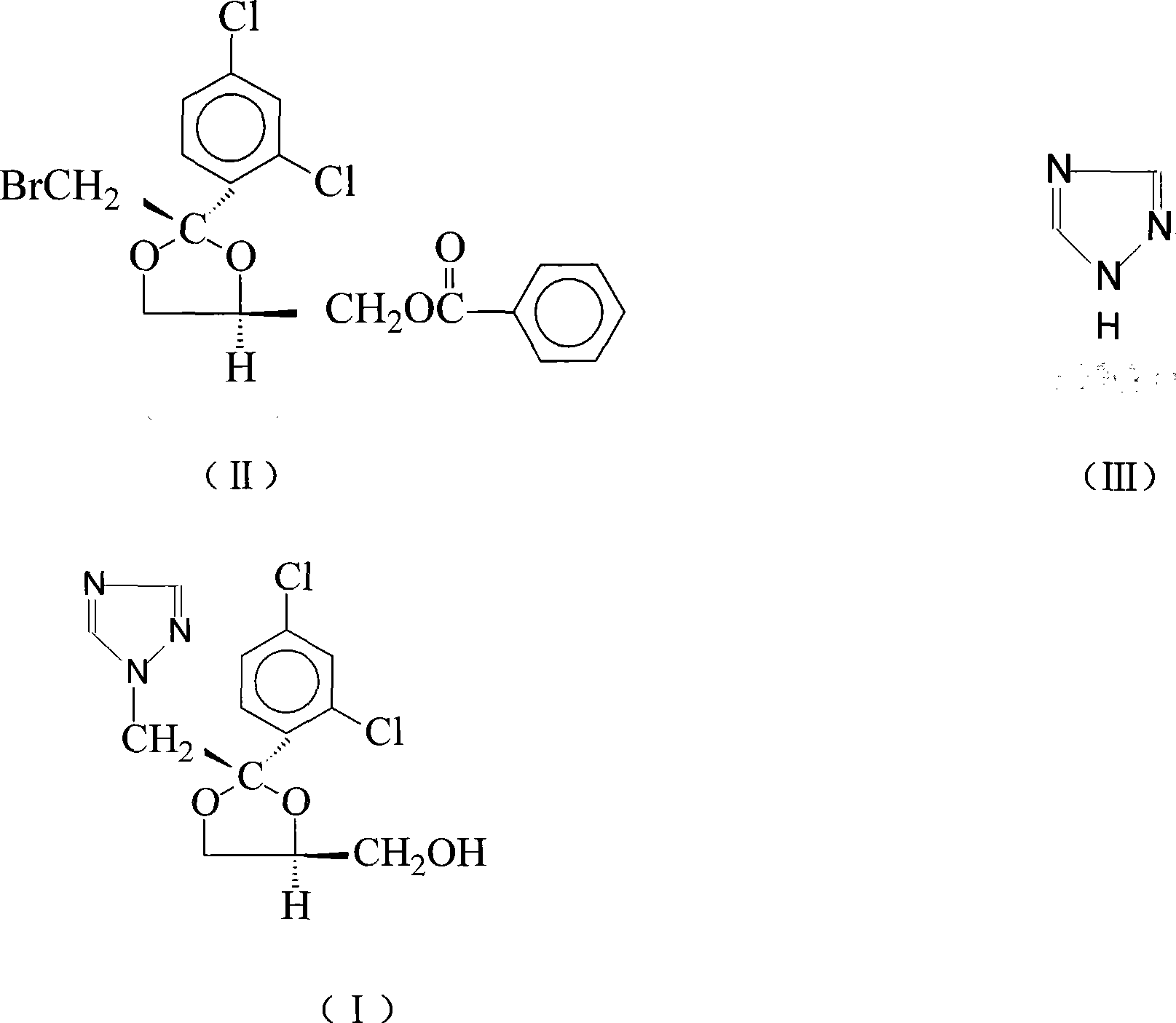
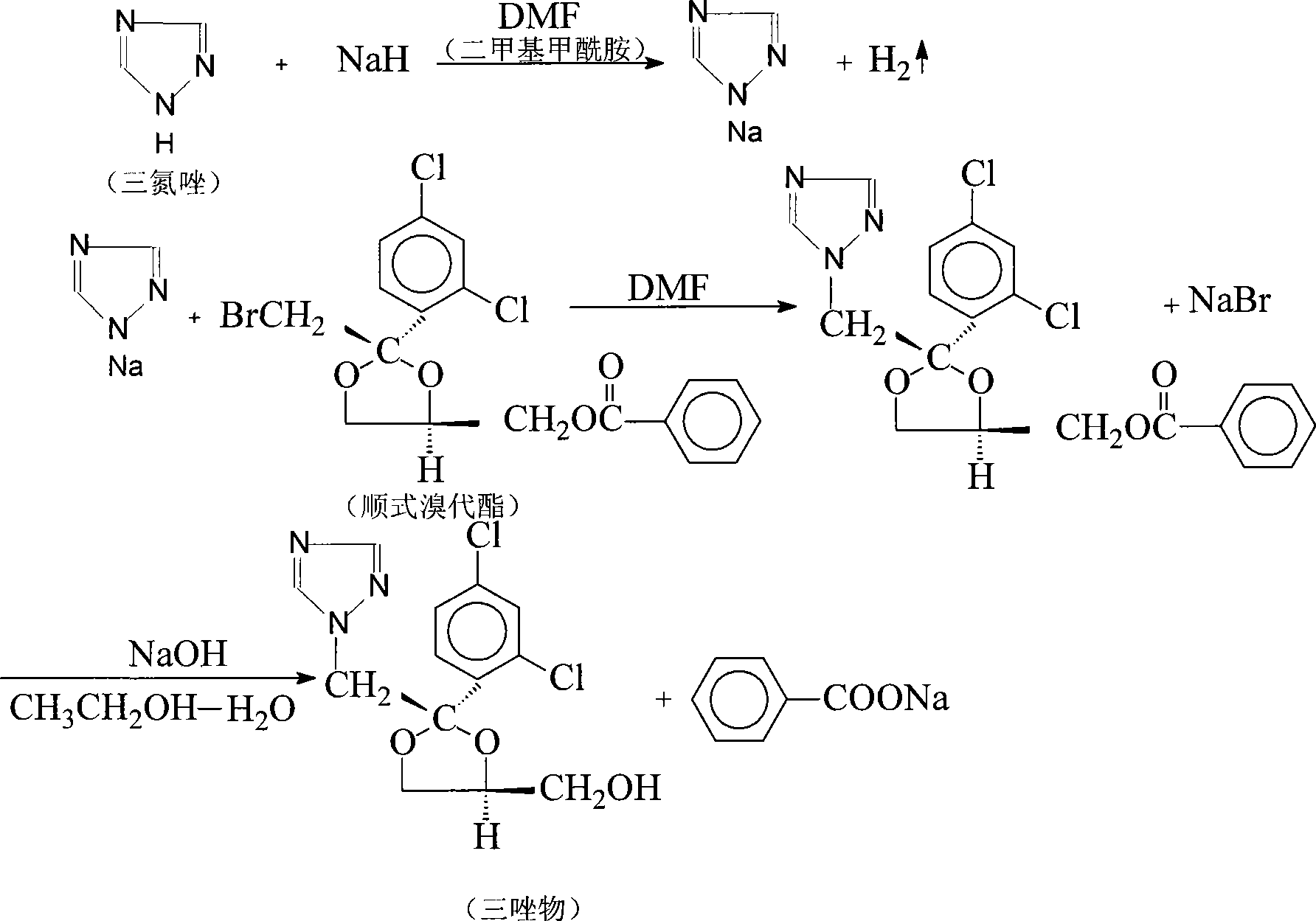
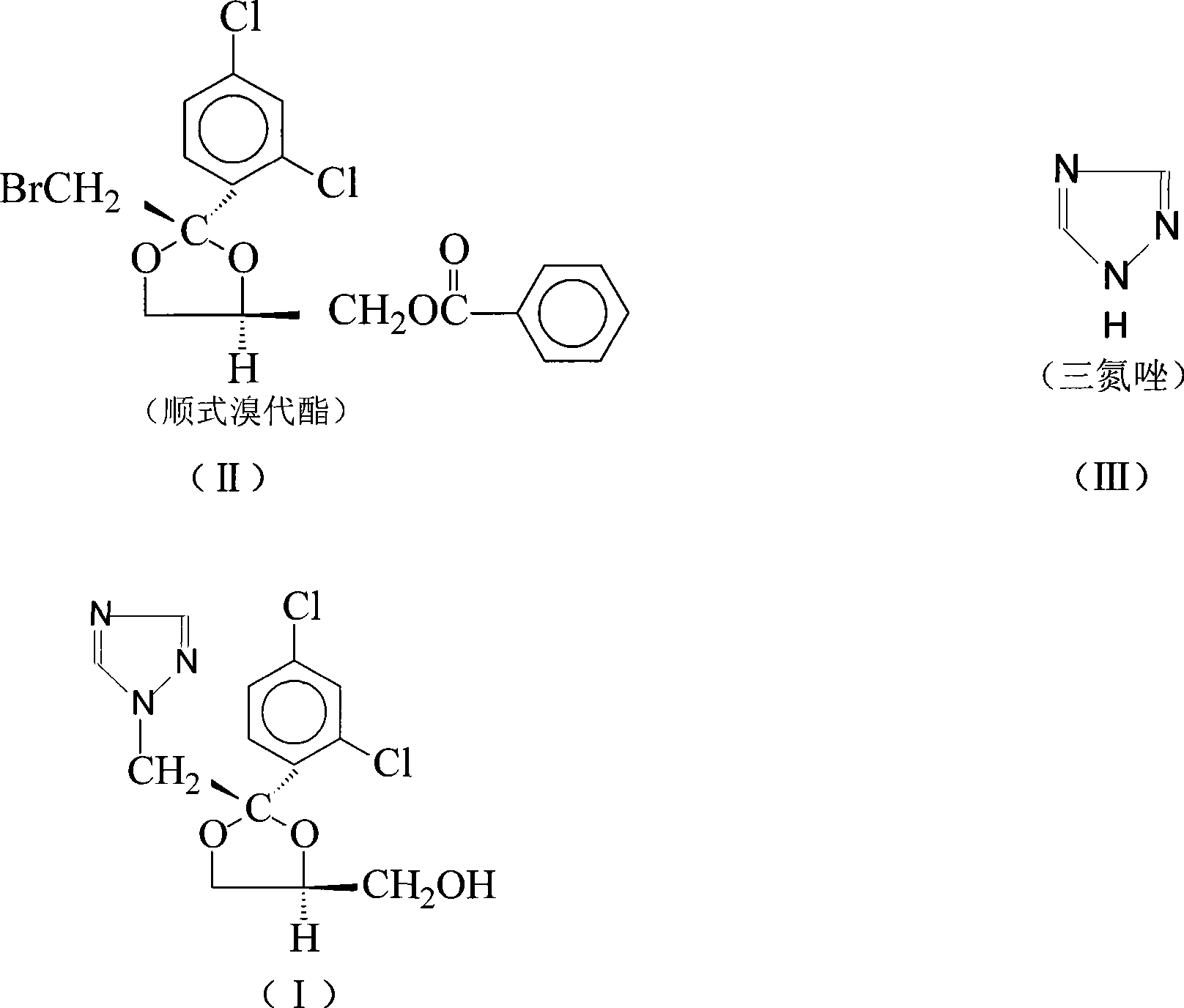
Synthetic method of itraconazole key intermediate triazole compounds
A synthetic method and technology of itraconazole, applied in the field of pharmaceutical chemical synthesis, can solve problems affecting the internal quality control of itraconazole products, complex and cumbersome process operations, high content of related substances, etc., and achieve market competitiveness and high yield High, short production cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 10g (0.022mol) cis bromoester, 11.8g (0.086mol) anhydrous potassium carbonate, 1.2g water, 6g (0.090mol) triazole, 0.1g polyethylene glycol 600, 50g respectively in the reaction flask Dimethyl sulfoxide, heated up to 189°C, reacted for 15 hours, and the reaction was complete. Add water, then extract with dichloromethane, separate the water layer, evaporate the dichloromethane in the organic layer until evaporated to dryness, add ethyl acetate for recrystallization, and obtain 6.1 g of "triazole" with a yield of 82.4%. The content is 87.60%, and the isomer content is 10.5%.
Embodiment 2
[0031] Add 10g (0.022mol) cis bromoester, 8.3g (0.06mol) anhydrous potassium carbonate, 0.1g water, 5g (0.075mol) triazole, 0.5g polyethylene glycol 400, 40g Dimethyl sulfoxide, heated up to 160°C, reacted for 20 hours, and the reaction was complete. Add water, then extract with chloroform, separate the water layer, evaporate the chloroform in the organic layer until it is evaporated to dryness, add ethyl acetate for recrystallization, and obtain 5.8 g of triazoles, the yield is 78.4%, and the content is 88.20%, isomer content 10.0%.
Embodiment 3
[0033] Add 10g (0.022mol) cis bromoester, 3.0g (0.022mol) anhydrous potassium carbonate, 1.5g water, 5g (0.075mol) triazole, 1g polyethylene glycol 800, 30g di Methyl sulfoxide, heated up to 140°C, reacted for 40 hours, and the reaction was complete. Add water, then extract with dichloromethane, separate the water layer, distill off the dichloromethane in the organic layer until evaporated to dryness, add ethyl acetate for recrystallization, and obtain 5.2 g of "triazole" with a yield of 70.3%. The content is 89.80%, and the isomer content is 9.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com