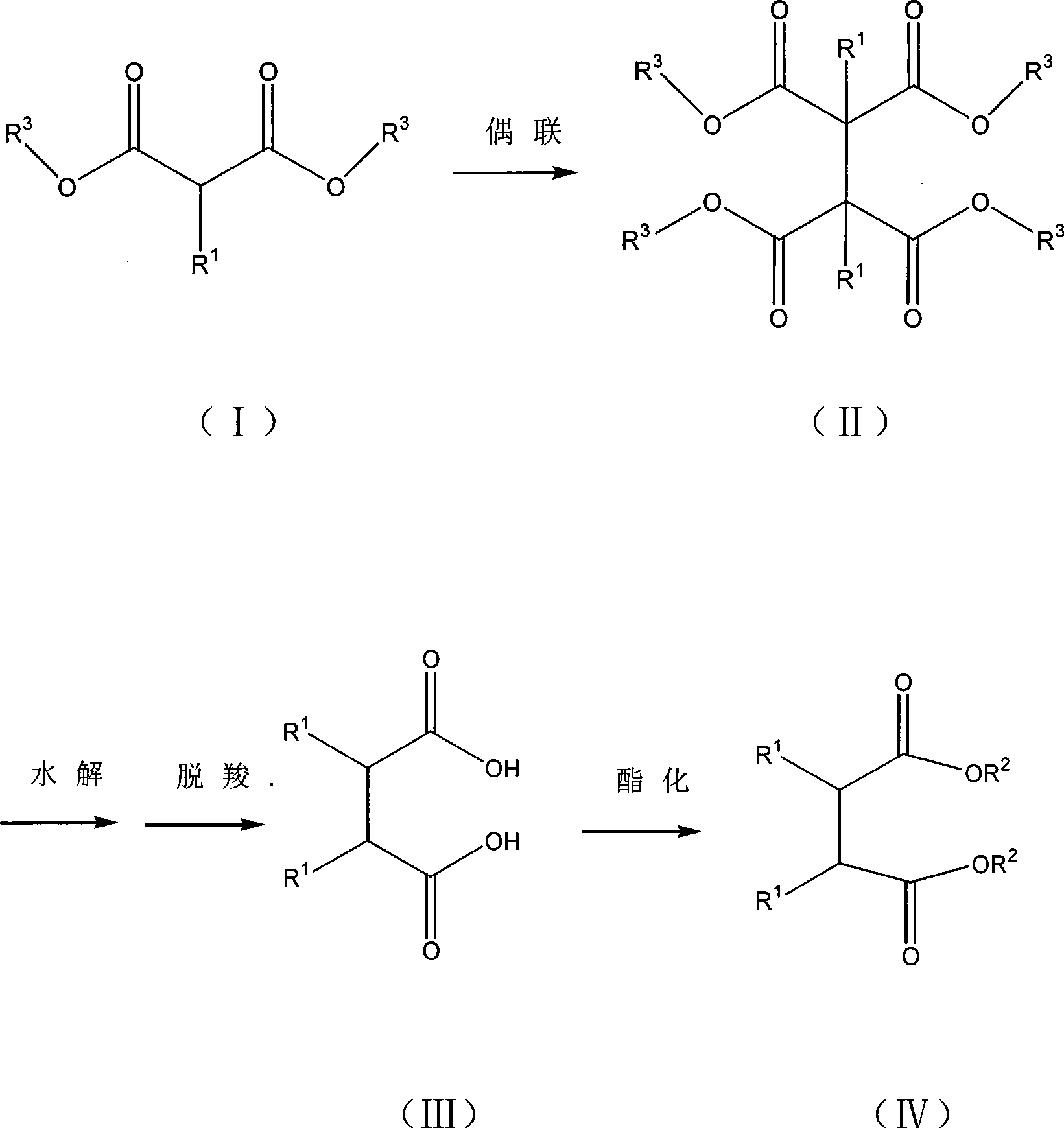
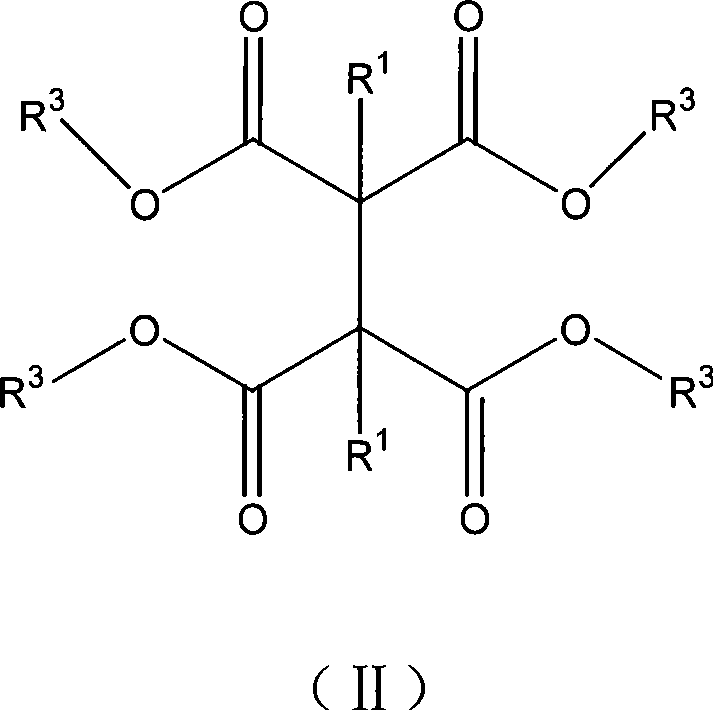
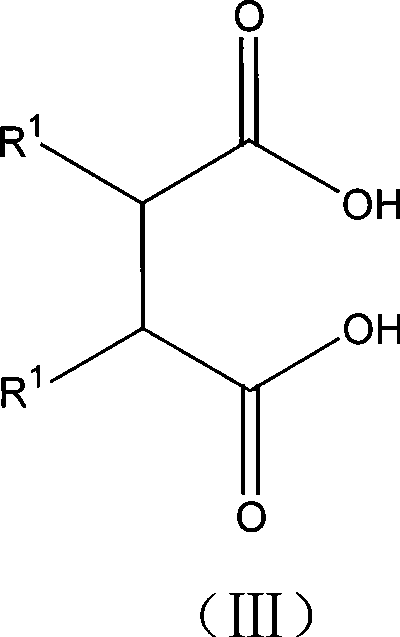
Method for preparing 2,3-dialkyl succinic acid and ester compounds thereof
A technology of dihydrocarbyl succinic acid and compounds, which is applied in two fields, and can solve problems such as not being disclosed, not being suitable for industrialization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0146] Examples 1-5, preparation of 1,2-dihydrocarbyl-1,1,2,2-tetrakis(alkoxycarbonyl)ethane.
Embodiment 1
[0147] Example 1: Preparation of 1,2-diphenyl-1,1,2,2-tetrakis(ethoxycarbonyl)ethane
[0148] Add 3.0 g of sodium hydride into a 250 ml one-necked flask. Then add 25ml of petroleum ether and shake to dissolve the mineral oil protecting sodium hydride in petroleum ether. Let it stand again to make the sodium hydride settle down. Pour out the petroleum ether, and then add 25ml petroleum ether into the one-mouth bottle. Wash like this three times. Then the residual petroleum ether was removed with a rotary evaporator. Remove the single-necked bottle, weigh 1.7 g of sodium hydride by differential gravimetric method, and quickly add 50 ml of dried tetrahydrofuran.
[0149] Weigh 11.8 g of diethyl phenylmalonate, and slowly add it dropwise into a single-necked bottle filled with sodium hydride. As the dropwise addition proceeded, bubbles emerged from the reaction solution. After the dropwise addition was completed, the reaction was continued for 1 hour to obtain a reaction sol...
Embodiment 2
[0155] Example 2: Preparation of 1,2-diphenyl-1,1,2,2-tetrakis(ethoxycarbonyl)ethane
[0156] Weigh 0.39 g of potassium metal by differential gravimetric method, and add it into 10 ml of absolute ethanol three times to obtain an ethanol solution of potassium ethoxide.
[0157] Add 2.36 g of diethyl phenylmalonate in a 100 ml one-necked flask, and then add 15 ml of absolute ethanol. Then, under the condition of stirring, the ethanol solution of potassium ethoxide was added dropwise into diethyl phenylmalonate with a constant pressure dropping funnel, and the reaction was carried out for 1 hour. Ethanol was then removed under reduced pressure to give a white solid. 15ml of dimethyl sulfoxide (DMSO) was added thereto, and the white solid was completely dissolved to obtain a reaction solution.
[0158] Weigh 1.27g of elemental iodine and dissolve it in 10ml of dimethyl sulfoxide (DMSO) to prepare an iodine solution. Then, at room temperature (about 20° C.), a DMSO solution of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com