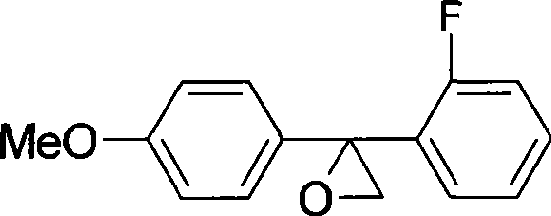
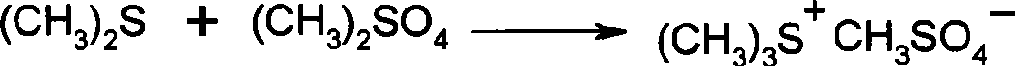Process for producing bactericide of flutriafol midbody
A technology for triazole alcohol and intermediates, applied in the field of preparation of intermediates, can solve the problems of low intermediate yield, large steric hindrance, difficulty in achieving quality and yield, etc., and achieves simple process, control of impurity content, The effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
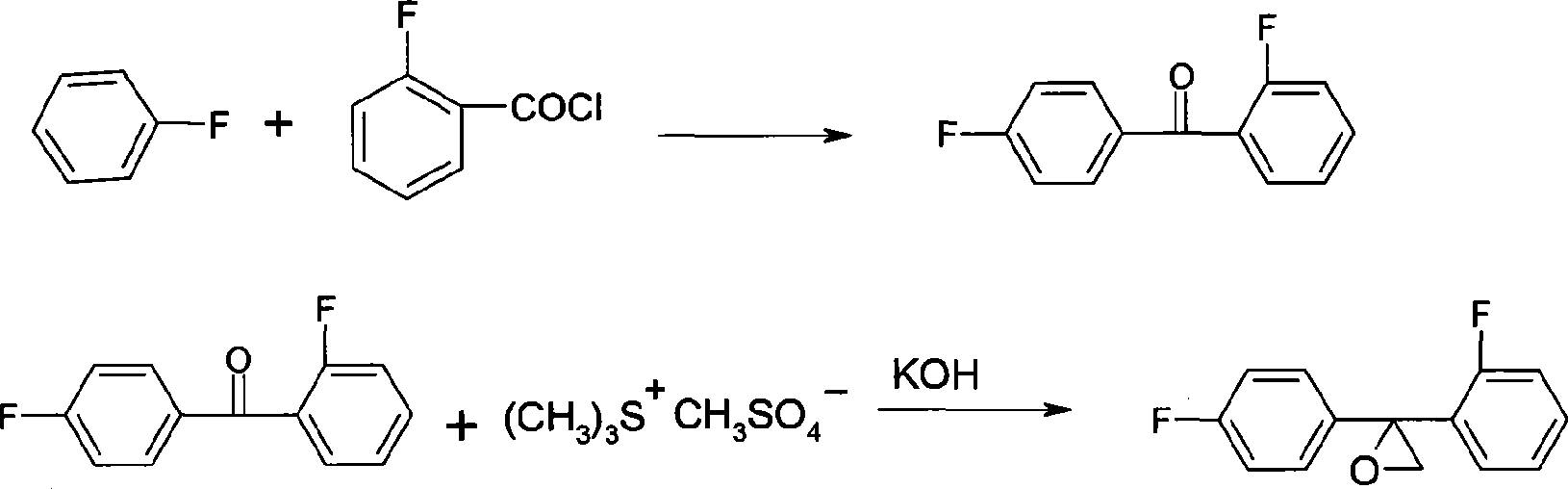
[0029] (1) Preparation of 2,4-difluorobenzophenone
[0030] 104g (99%, 1.08mol) fluorobenzene, 152g (99%, 1.14mol) AlCl 3 Put into 300g of nitrobenzene respectively, add 150g (99%, 0.95mol) o-fluorobenzoyl chloride dropwise, react at 20-50°C for 4h, after the reaction is completed, add water to separate layers, then wash with alkaline water until neutral, and depressurize The solvent was removed to obtain 190 g of 2,4-difluorobenzophenone with a content of 99%.
[0031] (2) Preparation of onium salt
[0032] 65g (99%, 1.05mol) of anhydrous dimethyl sulfide, 78g (99%, 1mol) of dimethyl sulfoxide, were respectively dropped into a 2L reaction flask equipped with a condenser tube, and 126g (99 %, 1mol) dimethyl sulfate, stirred for 4 hours, and then stood at room temperature overnight, and the obtained onium salt was directly used for the next reaction.
[0033] (3) Preparation of Triconazole Intermediate
[0034] In the above-mentioned reaction solution, add 600g toluene, the...
Embodiment 2
[0036] (1) Preparation of 2,4-difluorobenzophenone
[0037] 104g (99%, 1.08mol) fluorobenzene, 152g (99%, 1.14mol) AlCl 3 Put into 300g of fluorobenzene respectively, add 150g (99%, 0.95mol) o-fluorobenzoyl chloride dropwise, react at 20-50°C for 4h, after the reaction is completed, add water to separate layers, add alkaline water to wash until neutral, decompress to remove The solvent was removed to obtain 190 g of 2,4-difluorobenzophenone with a content of 99%.
[0038] (2) Preparation of onium salt
[0039] 65g (99%, 1.05mol) of anhydrous dimethyl sulfide, 50g (99%, 0.68mol) of tert-butanol are respectively dropped into a 2L reaction flask equipped with a condenser tube, and 126g (99 %, 1mol) dimethyl sulfate, stirred for 4 hours, and then stood at room temperature overnight, and the obtained onium salt was directly used for the next reaction.
[0040] (3) Preparation of Triconazole Intermediate
[0041] In above-mentioned reaction solution, add 600g pure benzene, then ...
Embodiment 3
[0043] (1) Preparation of 2,4-difluorobenzophenone
[0044] 104g (99%, 1.08mol) fluorobenzene, 152g (99%, 1.14mol) AlCl 3 Put into 300g of dichloroethane respectively, add dropwise 150g (99%, 0.95mol) o-fluorobenzoyl chloride, react at 20-50°C for 4h, after the reaction is completed, add water to separate layers, then add alkaline water to wash until neutral, reduce The solvent was removed by pressure to obtain 190 g of 2,4-difluorobenzophenone with a content of 99%.
[0045] (2) Preparation of onium salt
[0046] 65g (99%, 1.05mol) of anhydrous dimethyl sulfide, 60g (99%, 0.71mol) of dichloromethane were dropped into a 2L reaction flask equipped with a condenser tube respectively, and 126g (99 %, 1mol) dimethyl sulfate, stirred for 4 hours, and then stood at room temperature overnight, and the obtained onium salt was directly used for the next reaction.
[0047] (3) Preparation of Triconazole Intermediate
[0048]To the above reaction solution, add 600g of dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com