Alprostadil lipid complexes and micelle composition thereof for injection
A technology of dil lipid and alprostadil, which is applied in the direction of drug combination, drug delivery, and medical preparations of non-active ingredients, etc., can solve the problems of difficulty in normalizing the pharmacological activity and instability of alprostadil, etc., and achieve improved injection efficiency. Safety, reducing the inactivation of alprostadil, and increasing the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Take 10mg of alprostadil, 2.0g of egg yolk lecithin, 400mg of sodium cholesterol sulfate, and 10mg of vitamin E, and dissolve them in methanol at 30°C as the organic phase; dissolve 0.5g of PEG400 in 90ml of water for injection, and heat to 30°C as the water phase , inject the organic phase into the water phase under stirring at 30°C, continue to stir for 5 minutes, and then evaporate the methanol under reduced pressure to obtain the alprostadil lipid complex with blue opalescence and the micelle composition for injection thereof. Make up with water to a total volume of 100ml, and filter to sterilize with a 0.22μm microporous membrane.
[0043] Add 5% (w / v) trehalose to the obtained alprostadil lipoplex and the aqueous dispersion of the micelle composition for injection, freeze-dry under aseptic operation, and divide into packages to obtain.
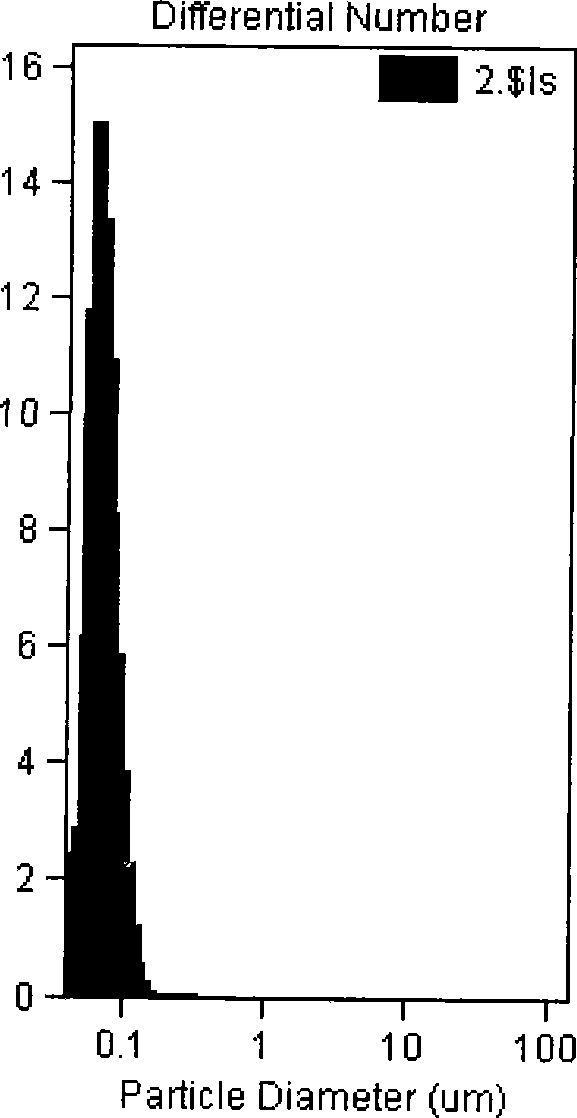
[0044] The lyophilized preparation was diluted with physiological saline, and its average particle diameter measured was 72.8 ± ...
Embodiment 2
[0046] Take 20mg of alprostadil, 3.0g of soybean lecithin, and 500mg of sodium cholesterol sulfate, and dissolve them in absolute ethanol at 35°C as the organic phase; dissolve 1.0g of PEG200 in 90ml of water for injection, heat to 35°C as the water phase, and Under stirring at 35°C, inject the organic phase into the water phase, continue to stir for 10 minutes, and then evaporate the ethanol under reduced pressure to obtain the alprostadil lipid complex with blue opalescence and its micelle composition for injection, which is replenished with water for injection. When the total volume reaches 100ml, it is sterilized by filtering through a 0.22μm microporous membrane, and then aliquoted.
Embodiment 3
[0048] Take 500 mg of alprostadil, 10 g of soybean lecithin, and 5 g of sodium cholesterol sulfonate, and dissolve them in tetrahydrofuran at 35 ° C as the organic phase; dissolve 5 g of poloxamer 188 in 950 ml of water for injection, heat to 35 ° C as the water phase, and Under stirring, inject the organic phase into the water phase, continue to stir for 10 minutes, and then evaporate the tetrahydrofuran under reduced pressure to obtain the alprostadil lipid complex with blue opalescence and the micelle composition for injection thereof, and add water for injection to make up to the total concentration. The volume is 1000ml, and it is sterilized by filtration through a 0.22μm microporous membrane.
[0049] Add 5% (w / v) trehalose to the obtained alprostadil lipoplex and the aqueous dispersion of the micelle composition for injection, freeze-dry under aseptic operation, and divide into packages to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com