Bimetal nano rod of branched gold core/platinum shell structure and preparation method thereof
A bimetallic nano, gold nanorod technology, applied in the coating and other directions, can solve the problems of gold and platinum nanorods that have not been reported, and achieve the effects of improving SERS properties, improving SPR characteristics, and improving poisoning phenomenon.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] First configure the following solutions:
[0047] 0.1M cetyltrimethylammonium bromide (C 19 h 42 N + Br - , hereinafter referred to as CTAB) aqueous solution: Weigh 3.64g of cetyltrimethylammonium bromide (analytical grade) and dissolve it in 100mL of deionized water; before use, it should be placed in a constant temperature water bath at 30°C to completely dissolve it.
[0048] 0.1M ascorbic acid (AA) aqueous solution: Weigh 0.176g of ascorbic acid (analytical pure) and dissolve it in 10mL of deionized water, and prepare temporarily before use.
[0049] 10mM tetrachloroauric acid (HAuCl 4 ·3H 2 O) Aqueous solution: Weigh 0.393g tetrachloroauric acid (analytical pure, domestic) and dissolve it in 100mL deionized water.
[0050] 10mM silver nitrate (AgNO 3 ) aqueous solution: weigh 0.425g of silver nitrate (analytical grade) and dissolve in 250mL of deionized water; store in the dark.
[0051] 2mM Potassium Platinous Chloride (K 2 PtCl 4 ) aqueous solution: Wei...
Embodiment 2
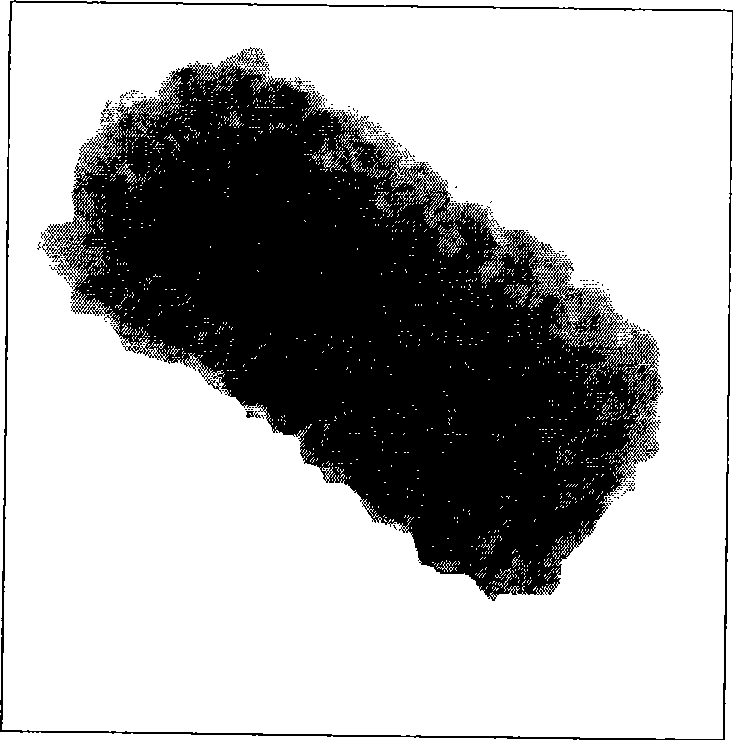
[0067] Get 2mL of the purified gold nanorod solution obtained in step 3 of Example 1 and put it into a test tube, and add 1mL of deionized water, 15.9uL of 10mM ascorbic acid aqueous solution (AA to platinum ion in dose ratio) and 79.6 uL 2mM potassium platinum chloride aqueous solution; mix well and put it in a constant temperature water bath at 30°C. After a few hours, the solution turns from maroon to gray-black, indicating the formation of a platinum shell structure; after 12 hours, the above reaction The solution was centrifuged at 12,000 rpm for 10 minutes; the supernatant was removed, the precipitate was diluted to the original volume with deionized water, and then centrifuged under the same conditions; so 2 to 4 times, the nanorods were separated from the reaction system to obtain gold Porous bimetallic nanorods with core / platinum shell structure. The resulting image with Figure 4 relatively close.
[0068] The molar ratio of ascorbic acid (AA) and potassium platini...
Embodiment 3
[0070] In this example, except that the volume of AA was selected as 79.6 μL (5 times excess of AA), the rest of the steps were the same as in Example 8, and the image of the obtained bimetallic nanorods with a gold core / platinum shell structure was similar to that in Example 2.
[0071] The molar ratio of ascorbic acid (AA) and potassium platinite chloride in the mixed solution described in this embodiment is 5:1; 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com