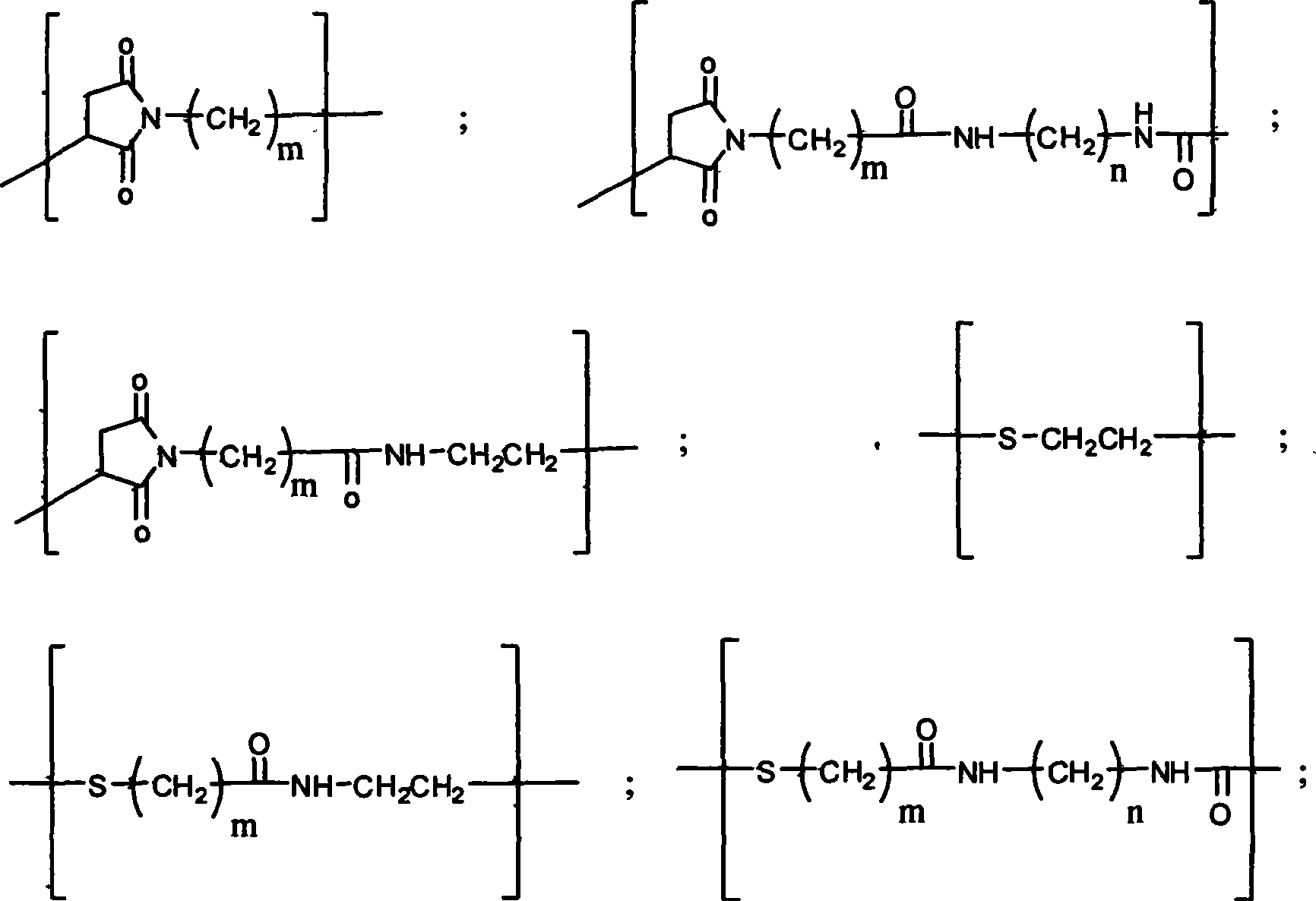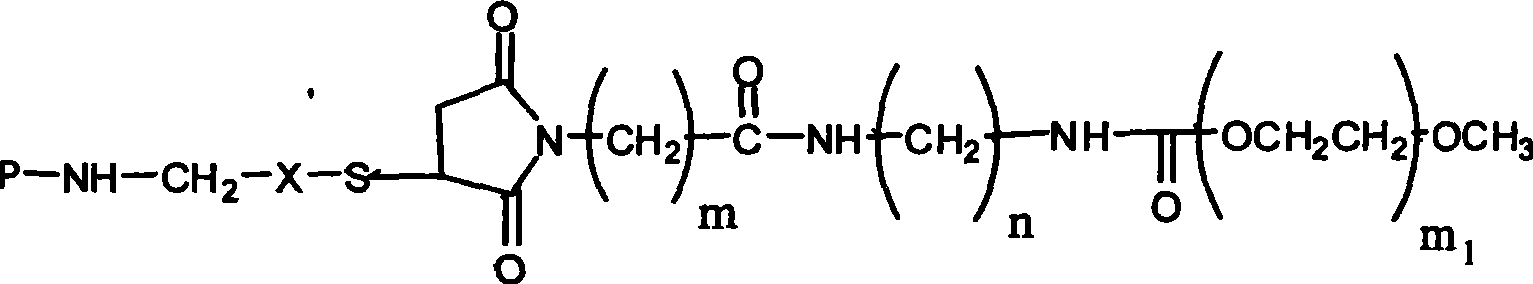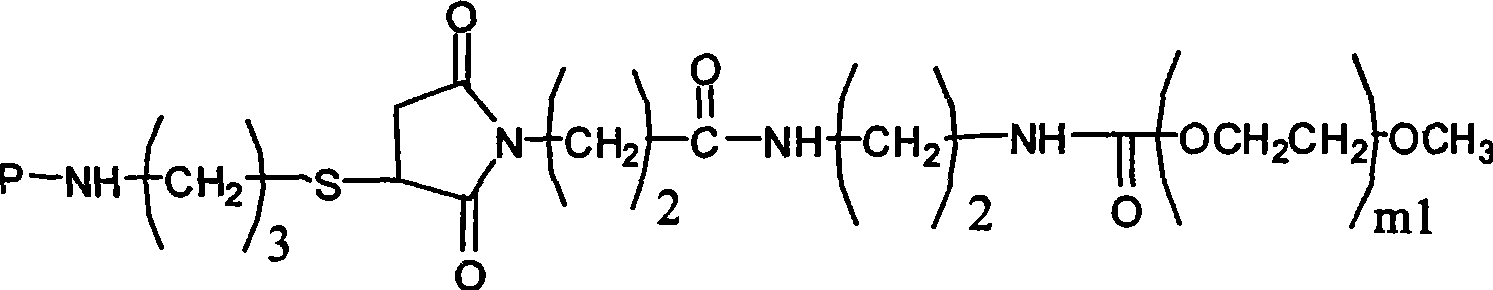PEG-erythrocyte-stimulating factor and preparation method and use thereof
A technology of erythropoietin and PEGylation, which is applied in the fields of erythropoietin, chemical instruments and methods, and extracellular fluid diseases, can solve the problems of protease degradation, short plasma half-life, and low utilization rate, and improve Residence time and circulation half-life, quality control, effect of reduced clearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of acetyl mercaptopropionaldehyde
[0077]
[0078] 11.2g (20mmol) of acrolein and dry 100ml of THF were added to the reaction flask, cooled to 0°C, and then slowly added dropwise a mixed solution of 1.52g (0.2mol) of thioacetic acid / 20ml of THF. After the dripping is completed, the reaction is kept for 2 hours. It was concentrated under reduced pressure at 35°C to remove excess acrolein. Then the column was quickly loaded (eluent pure n-hexane→n-hexane / ethyl acetate=50 / 1), the product spots were collected together, and concentrated under reduced pressure to dryness to obtain 0.6 g of an oily liquid.
Embodiment 2
[0079] Example 2: Preparation of mPEG-MAL-01 (20kD)
[0080]
[0081] Put 20g (1mmol) of mPEG-OH (20kD) into a 200ml single-necked flask, add 100ml of toluene, reflux and separate water for 2.5hr; then distill off the toluene, cool to room temperature, add 100ml of DCM, and then add 1.18g (4mmol) Triphosgene (triphosgene), the reaction was airtightly stirred overnight at room temperature; next day treatment: the reaction solution was washed in a fume hood into 200 ml of anhydrous ether, filtered and dried in vacuum to obtain 15 g of white solid. Put 15g of the white solid from the previous step into a 200ml single-necked flask, add 100ml of Toluene / DCM (2:1) solution, then add 0.25g of HOSu, and then add 0.3g of triethylamine, airtight and stir at room temperature for 4hr (or overnight) ); After the reaction is over, the reaction solution is filtered, and the filtrate is directly washed into 100ml of anhydrous ether, filtered, and vacuum dried to obtain 14g of white solid, which...
Embodiment 3
[0084] Example 3: Preparation of mPEG-MAL-02 (20kD)
[0085]
[0086] Dissolve 2.0 g of MAL-ONP in 50 mL DCM, add 0.05 g of triethylamine, and add 15 g of mPEG-NH 2 (20kD) was dissolved in 100ml of freshly opened DCM, and then added to the DCM solution of MAL-ONP, reacted overnight at room temperature; the next day, the DCM was concentrated under reduced pressure, and the residue was added to 200ml of anhydrous ether. The solid was precipitated by precipitation. After drying, 13 g of white solid is obtained, which is mPEG-MAL-02 (20kD).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com