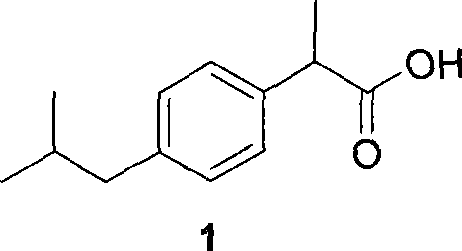
Method for preparing ibuprofen
A technology of isobutylphenyl and isobutylbenzene, which is applied in the synthesis field of medicine ibuprofen (propionic acid), and achieves the effects of reducing the amount of three wastes, high yield and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 300g of isobutylbenzene (excess) and 40.5g (0.3mol) of anhydrous aluminum chloride into a 500mL three-neck flask with a stirring and reflux tube, cool down to 0°C, and add 27.5g (0.35mol) of acetyl chloride dropwise while stirring, After 2 hours of addition, reflux reaction for 2 hours after the addition, cooling down after the reaction, pour the reaction solution into 500mL of ice water, separate the phases, and rectify the organic phase at normal pressure to recover 260g of excess isobutylbenzene to obtain 4-isobutylphenylethyl Ketone liquid 51.7g, content≥99.0% (gas chromatography, area normalization method).
[0029] Add 38g (0.30mol) of dimethyl sulfate, 20.0g (0.32mol) of dimethyl sulfide and 20)0mL of petroleum ether into a 500mL three-neck flask with a stirring and reflux tube, stir at 40°C for 2h, then add 4-isobutyl Base acetophenone liquid 52.0g (0.295mol) and potassium hydroxide 18g (0.32mol), reflux stirring reaction 7h, reaction finishes cooling, adds ...
Embodiment 2
[0031] Prepare 4-isobutyl acetophenone with example 1.
[0032] Add 40.5g (0.32mol) of dimethyl sulfate, 20.5g (0.33mol) of dimethyl sulfide and 200mL of petroleum ether into a 500mL three-necked flask with a stirring and reflux tube, stir at 40°C for 2h, then add 4-isobutyl 52g (0.295mol) of acetophenone liquid and 18g (0.32mol) of potassium hydroxide were refluxed and stirred for 9h. After the reaction was completed, the temperature was lowered, and hydrochloric acid was added for neutralization, phase separation, and the solvent was recovered by negative pressure distillation to obtain 2-(4-isobutyl phenyl)-1,2-propylene oxide yellow solid 55.2g, content≥98.8% (gas chromatography, area normalization method).
[0033] Add 38.1g (0.20mol) of 2-(4-isobutylphenyl)-1,2-propylene oxide, 0.4g of anhydrous zinc chloride, and 100mL of cyclohexanone into a 250mL three-necked flask with a stirring and drying tube, Stir at 0° C. for 4 h, after the reaction is complete, add 100 mL of w...
Embodiment 3
[0035] Prepare 4-isobutyl acetophenone with example 1.
[0036] Add 42.9g (0.34mol) of dimethyl sulfate, 23g (0.35mol) of dimethyl sulfide and 200mL of petroleum ether into a 500mL three-necked flask with a stirring and reflux tube, stir at 40°C for 2h, then add 4-isobutylbenzene 52g (0.295mol) of ethyl ketone liquid and 18g (0.32mol) of potassium hydroxide, refluxed and stirred for 10h, cooled down after the reaction, added hydrochloric acid for neutralization, phase separation, and vacuum distillation to recover the solvent to obtain 2-(4-isobutyl Phenyl)-1,2-propylene oxide yellow solid 55.4g, content ≥ 98.5% (gas chromatography, area normalization method).
[0037] Add 38.1g (0.20mol) of 2-(4-isobutylphenyl)-1,2-propylene oxide, 1.0g of anhydrous tin chloride, and 100mL of cyclohexanone into a 250mL three-necked flask with a stirring and drying tube, Stir at 20° C. for 2 h, after the reaction is complete, add 100 mL of water to wash and separate the phases. The organic ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com