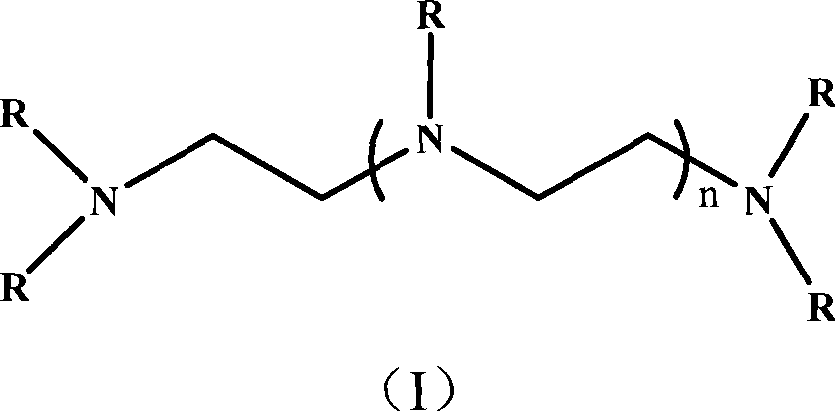
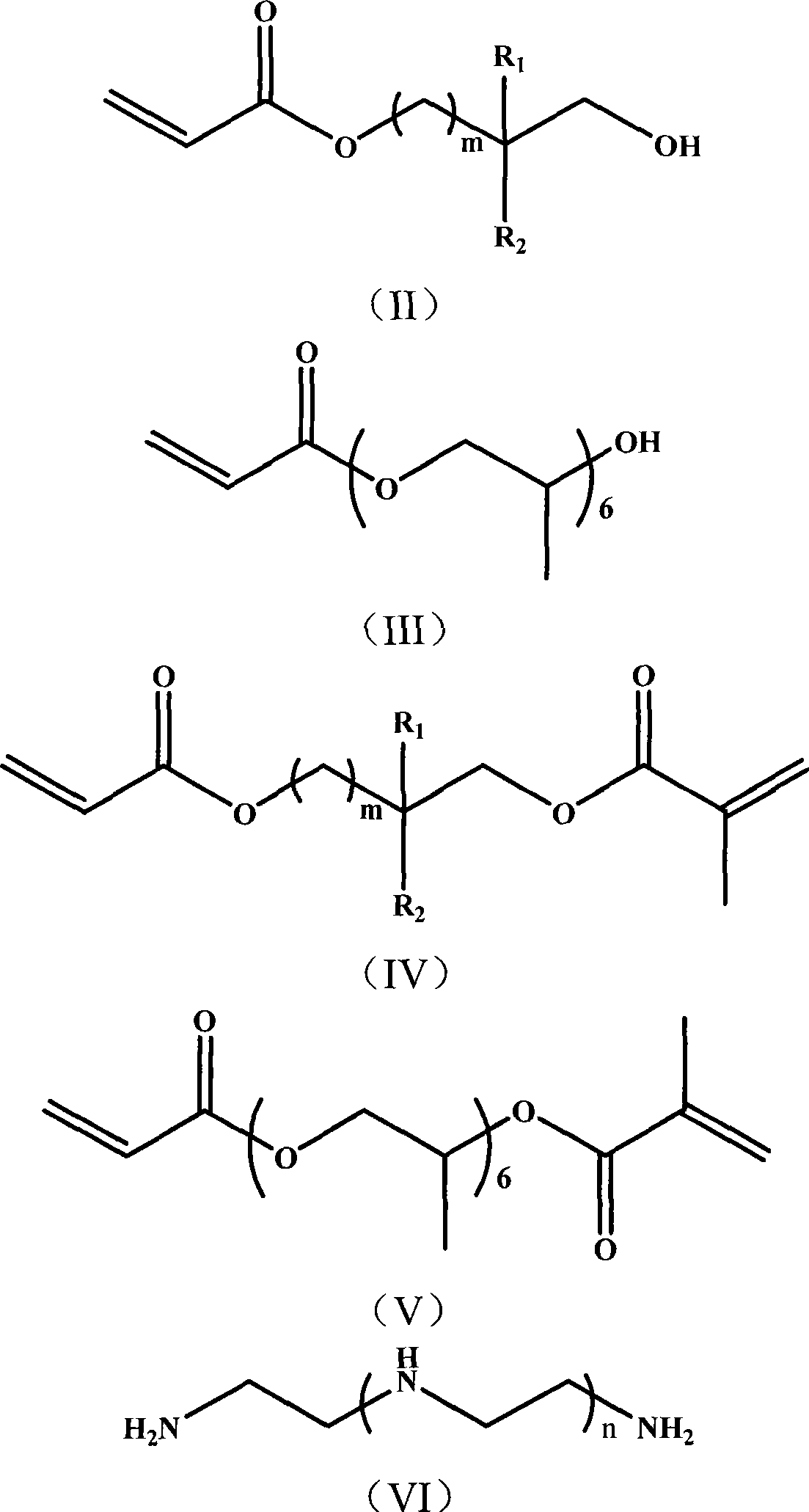
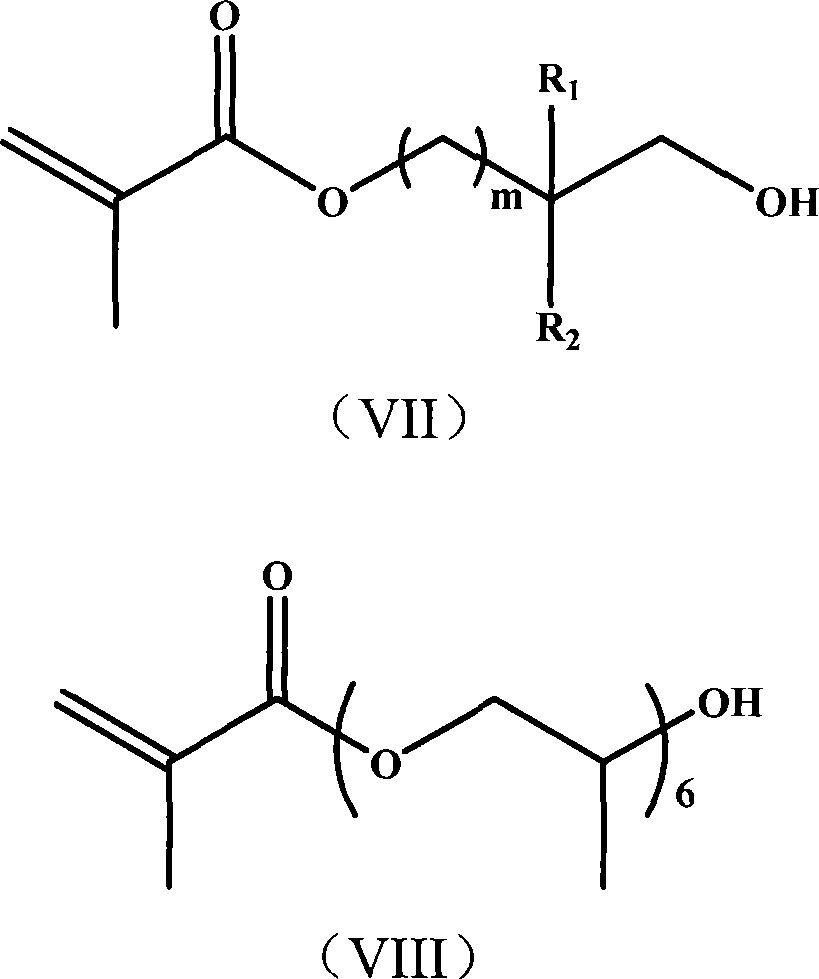
Nitrogen-containing polyfunctionality metacrylic acid ester monomer, preparation and use thereof
A methacrylate, multifunctional technology, applied in the preparation of cyanide reaction, chemical instruments and methods, and preparation of organic compounds, etc. Easy separation and purification of products, reduced stress shrinkage, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Add 34ml of methacryloyl chloride dropwise to a solution of 200ml of dichloromethane containing 46.45g of hydroxyethyl acrylate and 80g of triethylamine, and react in an ice bath at 0-5°C. After 4 hours of reaction, stop stirring , let stand overnight. After standing overnight, the reaction was terminated, and unreacted substances and by-products were removed by washing successively with water, 1M hydrochloric acid, and 1M NaOH solution. After removing the solvent, 65 g of acryloyloxyethyl methacrylate was obtained;
[0034] (2) 65 g of acryloyloxyethyl methacrylate was dissolved in 50 ml of methanol, and 5.29 g of ethylenediamine was added dropwise at -10°C. After the dropwise addition, continue to stir for 2 hours, then raise the temperature to 20°C and continue to stir at a constant temperature until the infrared detection 1640 and 810cm -1 The left and right double bond absorption peaks disappear, the reaction is stopped, and solvent methanol is removed to obta...
Embodiment 2
[0041] (1) Add 43ml of methacryloyl chloride dropwise to 300ml of ethyl acetate solution containing 58.06g of hydroxyethyl acrylate and 110g of tripropylamine, and react in an ice bath at 0-5°C. After 4 hours of reaction, stop stirring, Let stand overnight. After standing overnight, the reaction was terminated, and unreacted substances and by-products were removed by washing successively with water, 1M hydrochloric acid, and 1M NaOH solution. After removing the solvent, 81.25 g of acryloyloxyethyl methacrylate was obtained;
[0042](2) 81.25 g of acryloyloxyethyl methacrylate was dissolved in 50 ml of methanol, and 9.10 g of diethylenetriamine was added dropwise at 0°C. After the dropwise addition, continue to stir for 2 hours, then raise the temperature to 25°C and continue to stir at a constant temperature until the infrared detection 1640 and 810cm -1 The left and right double bond absorption peaks disappeared, the reaction was stopped, and the solvent methanol was removed...
Embodiment 3
[0049] (1) Add 61ml of methacryloyl chloride dropwise to 500ml of n-hexane solution containing 81.28g of hydroxyethyl acrylate and 175g of tri-n-butylamine, and react in an ice bath at 0-5°C. After 4 hours of reaction, stop Stir and let stand overnight. After standing overnight, the reaction was terminated, and unreacted substances and by-products were removed by washing successively with water, 1M hydrochloric acid, and 1M NaOH solution. After removing the solvent, 113 g of acryloyloxyethyl methacrylate was obtained;
[0050] (2) 113 g of acryloyloxyethyl methacrylate was dissolved in 50 ml of methanol, and 16.60 g of tetraethylenepentamine was added dropwise at -10°C. After the dropwise addition, continue to stir for 2 hours, then raise the temperature to 50°C and continue to stir at a constant temperature until the infrared detection 1640 and 810cm -1 The left and right double bond absorption peaks disappeared, the reaction was stopped, and the solvent methanol was removed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com