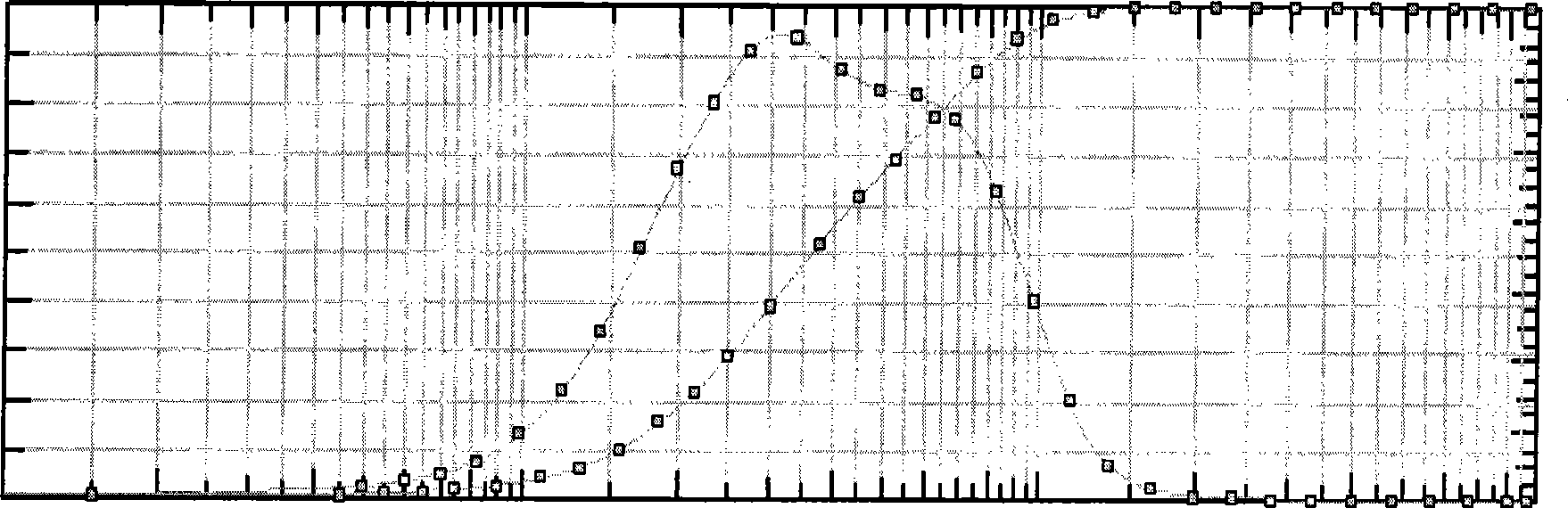
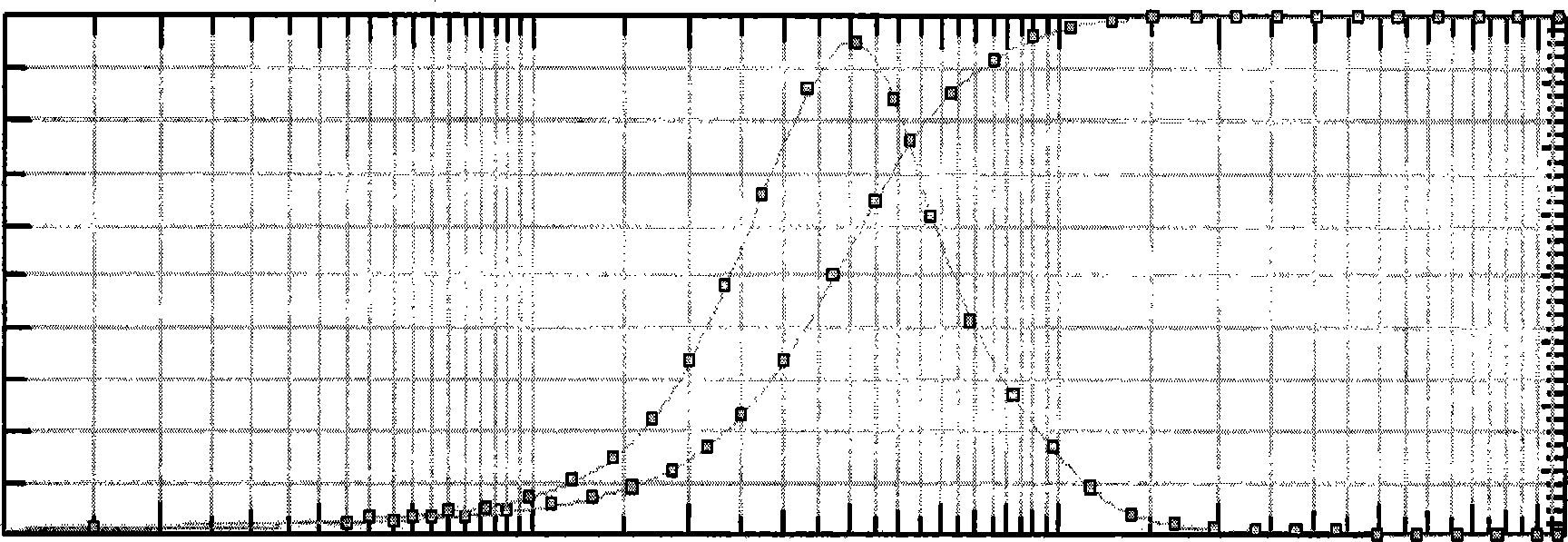
Gastrodine nosal spray preparation
A gastrodin and preparation technology, applied in the field of gastrodin nasal spray preparations, can solve the problems of increasing patient pain, side effects, and aggravating the mental burden of patients with nervous system diseases, so as to improve drug efficacy, high bioavailability, and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Formula: gastrodin 1g; Tween 20: 0.024g; polyethylene glycol 400: 192g; propylene glycol: 48g; EDTA disodium salt: 0.1g; benzalkonium chloride (50%): 0.3g; Make up to 1000g with water;
[0110] Preparation:
[0111] (1) Dissolve Tween 20 in 200g of water, shear and mix, add disodium edetate, benzalkonium chloride, and mix;
[0112] (2) Get 300g water and add gastrodin, propylene glycol, polyethylene glycol 400 successively and stir and mix;
[0113] (3) Add the mixed solution in (1) to (2), add water to 1000g in sufficient amount, stir and mix to obtain a clear, stable and transparent solution.
[0114] (4) Adjust pH to 6.5 with HCl (1mol / l) and NaOH (2mol / l) solution to obtain 1000g of clear, transparent, colorless, bubble-free fluid.
[0115] (5) Filling, dispensing and sealing the fluid obtained in (4) into a spray bottle. Each bottle of nasal spray contains 16.5g gastrodin nasal spray.
Embodiment 2
[0117] Formula: Cannabinoid 1g; Tween 20: 0.024g; Polyethylene glycol 400: 192g; Propylene glycol: 48g; Ethylenediamine
[0118] Disodium tetraacetate: 0.1g; benzalkonium chloride (50%): 0.3g; pure water to make up to 1000g;
[0119] The preparation method is the same as in Example 1.
Embodiment 3
[0121] Formula: Gastrodin 2g; Tween 20: 0.024g; Polyethylene glycol 400: 192g; Propylene glycol: 48g; EDTA disodium salt: 0.1g; Benzalkonium chloride (50%): 0.3g; Make up to 1000g with water;
[0122] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com