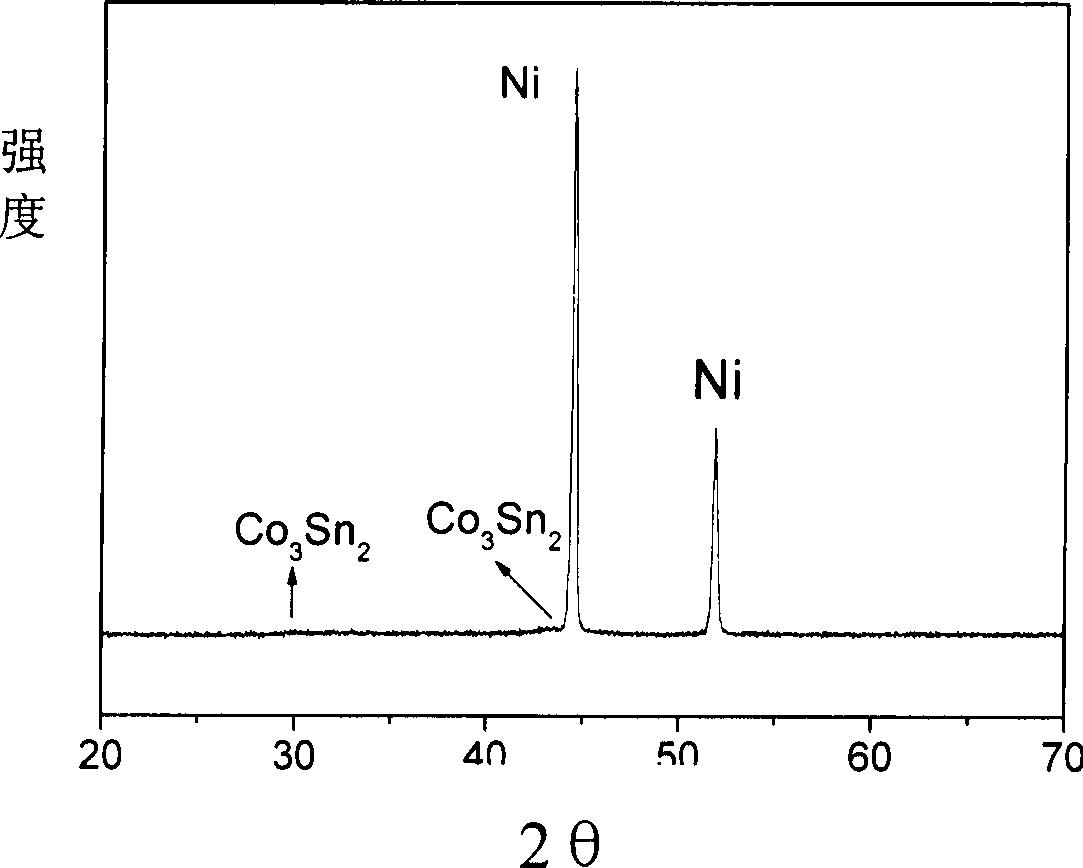
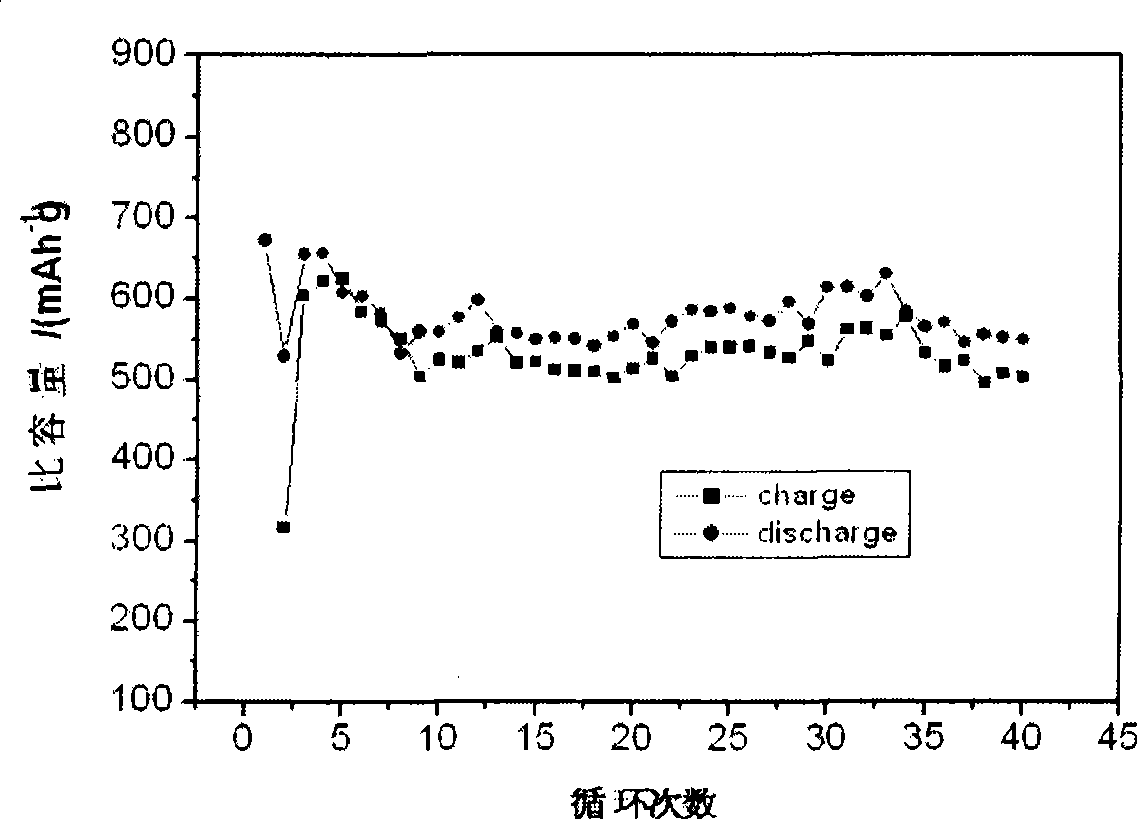
Electrochemical deposition preparation for lithium ionic cell tin-cobalt alloy film electrode
A lithium-ion battery, tin-cobalt alloy technology, applied in battery electrodes, alkaline battery electrodes, circuits, etc., can solve the problems of complex preparation process and large volume expansion of polystyrene balls, and achieve good cycle performance, high capacity, The effect of mitigating the effect of volume expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of electrolyte solution of tin-cobalt alloy
[0032] a. In a 100ml beaker, dissolve 15g of SnCl with 20ml of pure hydrochloric acid 2· 2H 2 O is heated and dissolved, and diluted to 100ml after dissolution;
[0033] b. In a 100ml beaker, heat and dissolve 15g CoCl with 100ml distilled water 2· 6H 2 O;
[0034] c. In a 500ml beaker, heat and dissolve 200g K with 200ml distilled water 4 P 2 o 7 .
[0035] d. First add the SnCl2 solution to the K 4 P 2 o 7 solution, and then CoCl 2 solution added to K 4 P 2 o 7 Then add 5g of L-methionine to the solution, adjust the pH of the solution to 7-9 with ammonia water, and finally set the volume to 500ml with a volumetric flask to obtain a purple tin-cobalt electrolyte.
[0036] 2. The electrochemical deposition system is composed of nickel foam as cathode, graphite electrode as anode, DC power supply and electrolyte solution containing tin-cobalt metal ions;
[0037] 3. Electrochemical deposition is...
Embodiment 2
[0043] 1. Preparation of electrolyte solution of tin-cobalt alloy
[0044] a. In a 100ml beaker, dissolve 15g Na with 20ml pure hydrochloric acid 2 SnO 3· 3H 2 O is heated and dissolved, and diluted to 100ml after dissolution;
[0045] b. In a 100ml beaker, heat and dissolve 15g CoSO with 100ml distilled water 4· 7H 2 O;
[0046] c. In a 500ml beaker, heat and dissolve 200g K with 200ml distilled water 4 P 2 o 7 .
[0047] d. Na 2 SnO 3 solution added to K 4 P 2 o 7 solution, and then Co(NO 3 ) 2 solution added to K 4 P 2 o 7 Then add 5g of hydroxylammonium hydrochloride, adjust the pH of the solution to 7-9 with ammonia water, and finally set the volume to 500ml with a volumetric flask to obtain a purple tin-cobalt electrolyte.
[0048] 2. The electrochemical deposition system is composed of nickel foam as cathode, graphite electrode as anode, DC power supply and electrolyte solution containing tin-cobalt metal ions;
[0049] 3. Electrochemical deposition ...
Embodiment 3
[0052] 1. Preparation of electrolyte solution of tin-cobalt alloy
[0053] a. In a 100ml beaker, dissolve 15g of SnCl with 20ml of pure hydrochloric acid 2· 2H 2 O is heated to dissolve, and diluted to 100ml after dissolution.
[0054] b. In a 100ml beaker, heat and dissolve 15g CoCl with 100ml distilled water 2· 6H 2 O;
[0055] c. In a 500ml beaker, heat and dissolve 200g K with 200ml distilled water 4 P 2 o 7 ;
[0056] d. SnCl first 2 solution added to K 4 P 2 o 7 solution, and then CoCl 2 solution added to K 4 P 2 o 7 Then add 5gL-methionine to the solution, adjust the pH of the solution to 7-9 with ammonia water, and finally set the volume to 500ml with a volumetric flask to obtain a purple tin-cobalt electrolyte.
[0057] 2. The electrochemical deposition system is composed of nickel foam as cathode, graphite electrode as anode, DC power supply and electrolyte solution containing tin-cobalt metal ions;
[0058] 3. Electrochemical deposition is carried o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com