Sulphonate Gemini surfactant and preparation method thereof
A surfactant, gemini surface technology, applied in chemical instruments and methods, dissolution, chemical/physical processes, etc., can solve the problem of limited types of gemini surfactants, not many, etc., to achieve good slime peeling characteristics, easy to operate , excellent surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Further illustrate the present invention below by EM-12 synthesis example
[0032] 1. Ring-opening reaction of maleic anhydride
[0033] At room temperature, use argon to exhaust the air in a dry 250mL three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser, add 5mL of acetone as a solvent, first add 37.5g of maleic anhydride, and then dropwise add ethylenediamine 15.6 mL, the molar ratio of the amount used is 2.5:1, and then the catalyst BF is added dropwise 3 -Ether solution (w=47%) 2mL, heated and stirred to reflux under the protection of argon, reacted for 18h, washed the crude product with acetone for 3-5 times, filtered with suction, and dried in vacuum at 60°C to obtain light yellow intermediate I Ethylamine bis(acyl-acrylic acid) powder 35.9g, yield rate is 87.75%;
[0034] 2. Addition reaction
[0035] In a dry 250mL three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser, add 10g of intermediate I, dropwis...
Embodiment 2
[0039] Surface Activity Determination of EM Series Surfactants
[0040] The surface tension of the product solution under different concentrations is measured by the suspension ring method, and the surface tension of the EM-12 aqueous solution is changed with the concentration curve, see Figure 4 . The critical micelle concentration value (cmc) and the surface tension (γ cmc ). Experiments have found that the critical micelle concentration of EM series surfactants and the surface tension at the critical micelle concentration are low. For example, the critical micelle concentration of EM-12 is 0.42mmol / L, and the surface tension at the critical micelle concentration is 28.8 mN / m.
Embodiment 3
[0042] Infrared spectrum of intermediate products and target products EM series surfactants
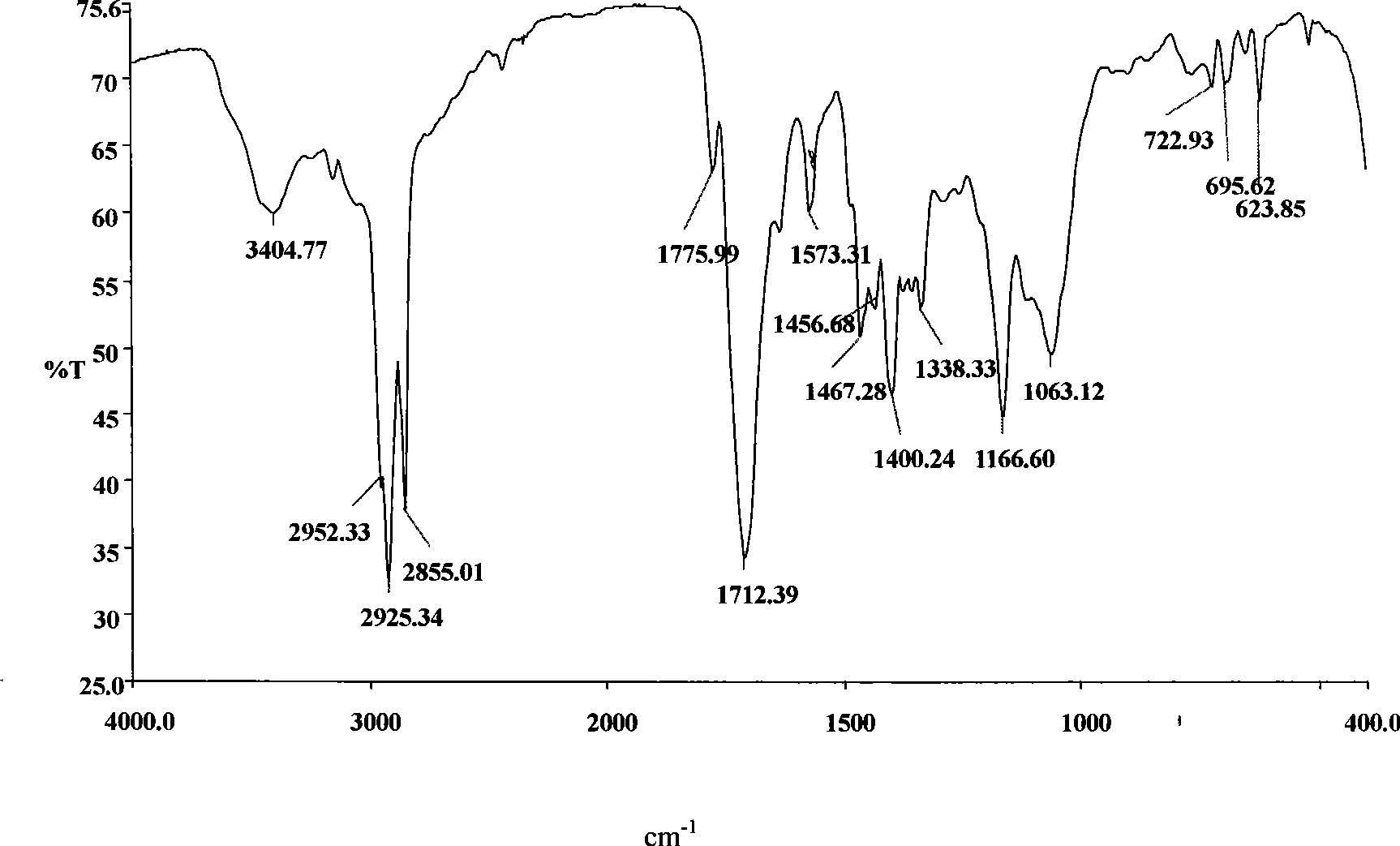
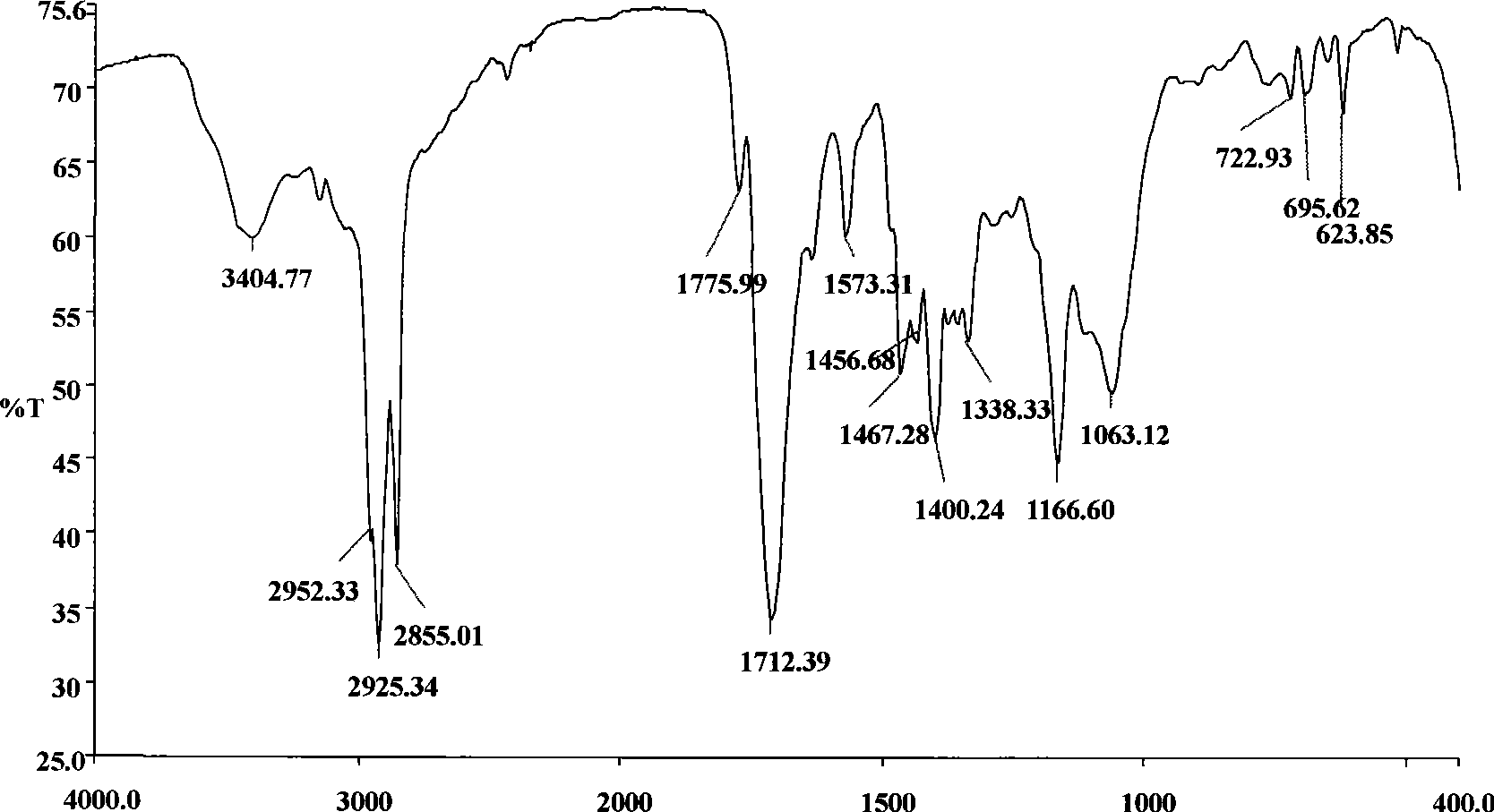
[0043] The infrared spectrum of the intermediate obtained by example 1, see figure 1 , 2 ; Infrared spectrum of EM-12 surfactant see image 3 , from the spectrum analysis we know:
[0044] figure 1 , intermediate product I: 3294.26cm -1 It is the stretching vibration peak of N-H; 3065.47cm -1 It is the stretching vibration peak of C-H; 2850.21cm -1 for -CH 2 The antisymmetric and symmetric stretching vibration peaks of -; 1697.21cm-1 is the -C=O stretching vibration peak in α, β unsaturated acids; 1629.68cm-1 It is -N-C=O stretching vibration peak; 1582.36cm -1 It is the bending vibration peak of secondary amine; 715.19cm -1 for -CH 2 stretching vibration peak.
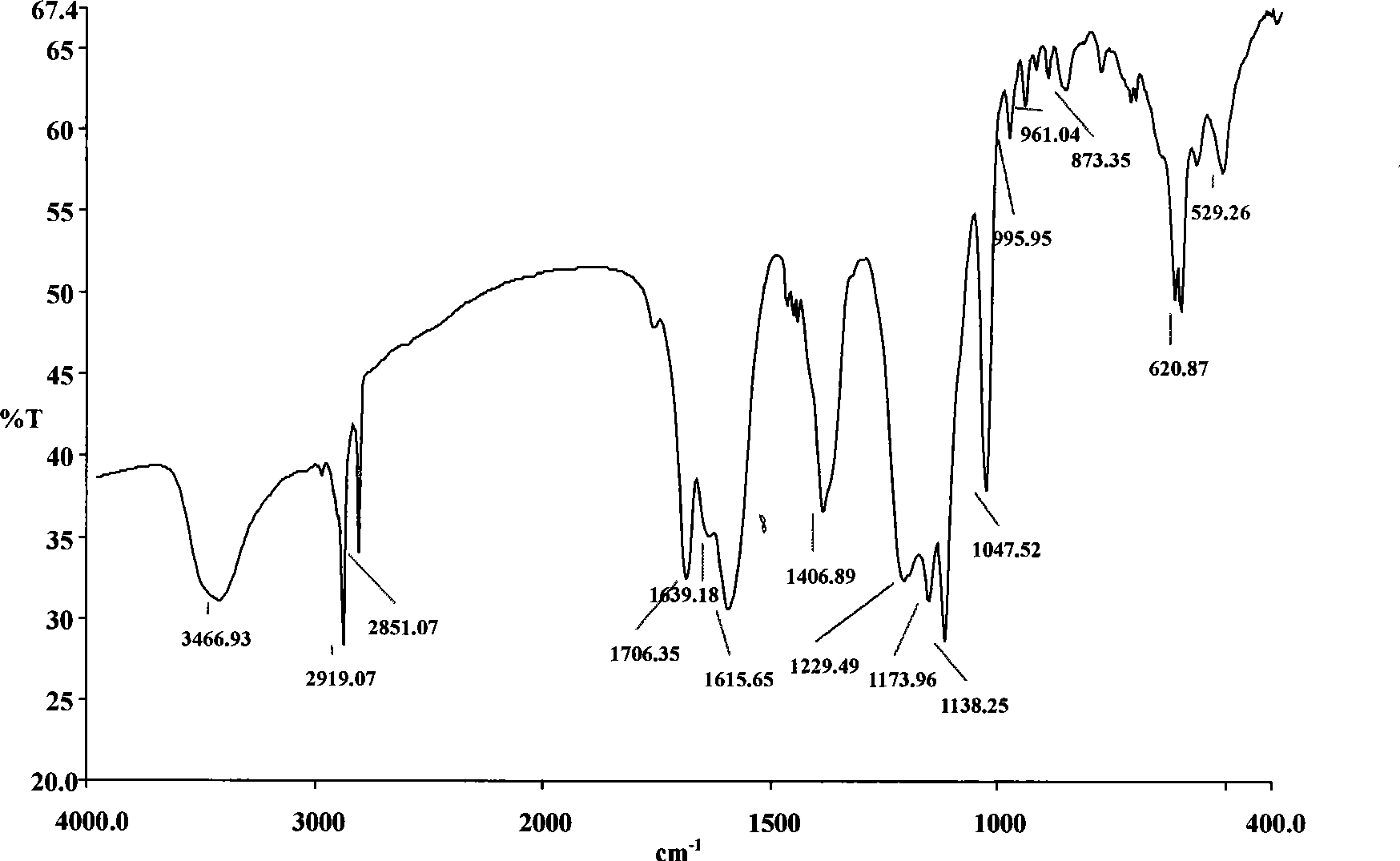
[0045] figure 2 , intermediate product II: 3466.93cm -1 It is the stretching vibration peak of N-H; 2919.07 and 2851.07cm -1 for -CH 2 The antisymmetric and symmetric stretching vibration peaks of ; 1706.35cm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com