Continuous production method of epoxy chloropropane by hydrogen peroxide process
A technology of epichlorohydrin and its production method, which is applied in the production field of epichlorohydrin, can solve the problems of increasing catalyst regeneration frequency and cost, cost increase, and large loss of allyl chloride, and achieve activity and epoxidation selectivity maintenance , Improve the unit production capacity, and the effect of smooth operation control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
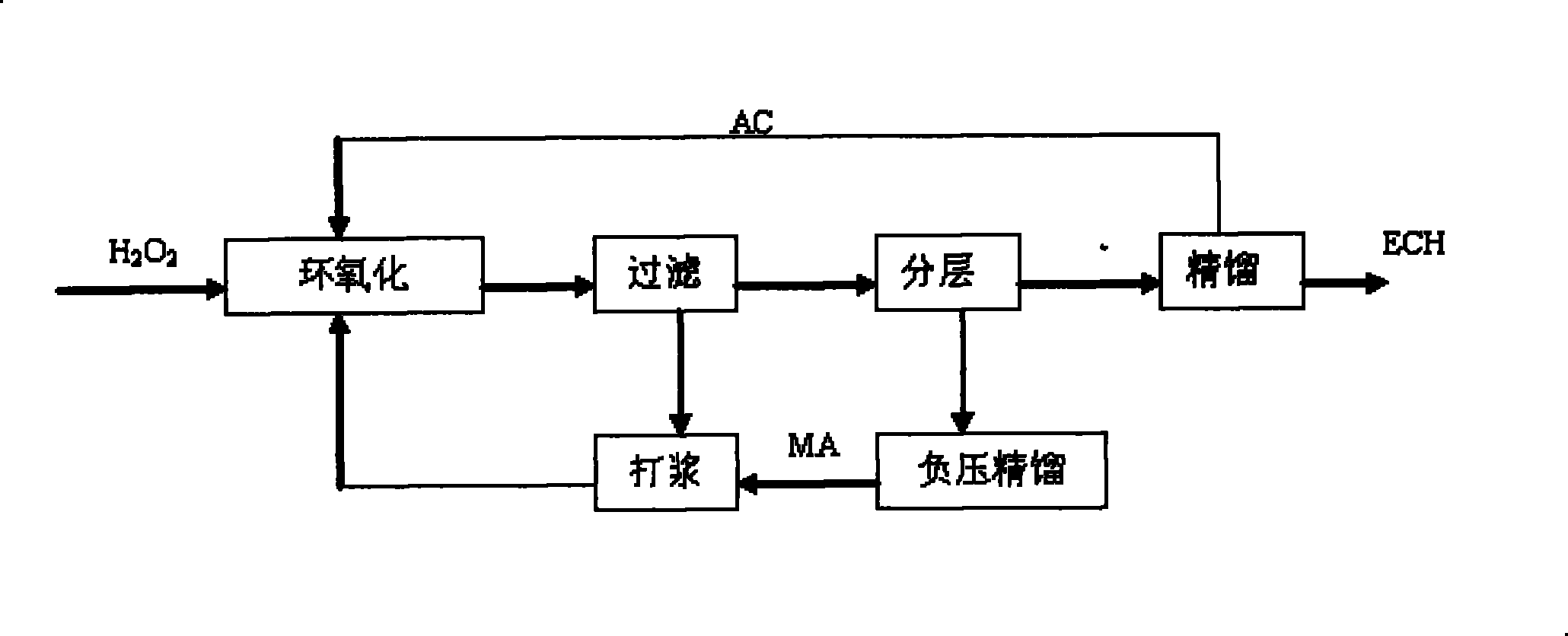
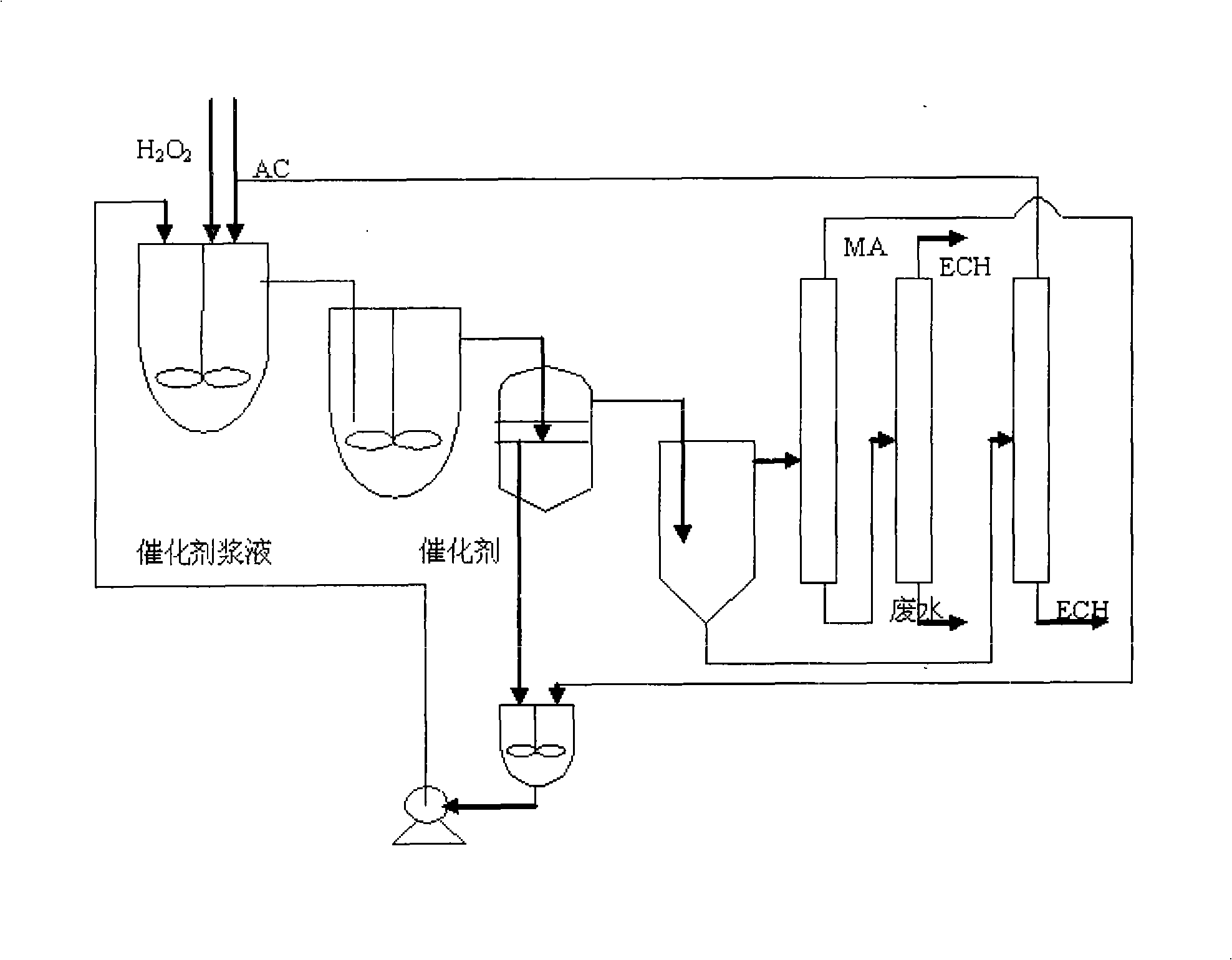
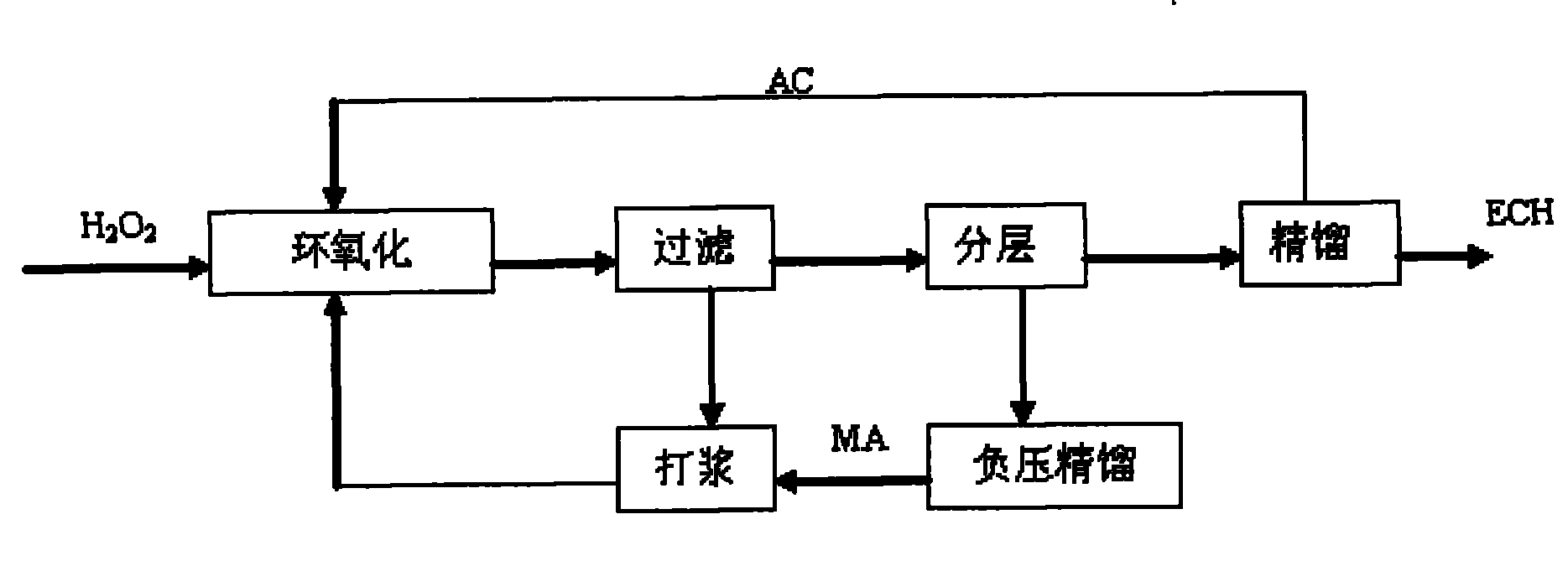
[0021] Three kettles are connected in series, the effective volume of each kettle: 130mL, 270mL, 230mL, the total effective volume is 630mL. The reaction temperature is 41-49℃, the reaction is exothermic reaction, and the heat is exchanged through the jacket. Catalyst with 27.5% H 2 o 2 The mass ratio of 1:12. Allyl chloride (AC) and H 2 o 2 Use a peristaltic pump to drive into the first-stage reaction kettle at the same time, and the flow rates are V AC =167.1mL / hr, V H2O2 =87.0mL / hr, methanol and catalyst are mixed evenly and beating with peristaltic pump to control feeding, V MA =165.9 mL / hr. The stirring speed is 350rpm, the three reactors stay for about 1.5hr, the feed flow rate per hour is 420mL / h, and the reaction materials overflow from the upper end of the reactor and enter the next reactor.
[0022]After the reaction is over, the aqueous phase catalyst slurry is separated by membrane filtration, and the catalyst filter cake or concentrated slurry is directly b...
Embodiment 2
[0027] Three kettles are connected in series, the effective volume of each kettle: 130mL, 270mL, 230mL, the total effective volume is 630mL, the reaction temperature is 41-49℃, the reaction is exothermic reaction, and the heat is exchanged through the jacket. Catalyst with 35% H 2 o 2 The mass ratio is 1:10. AC and H 2 o 2 Use a peristaltic pump at a certain flow rate to drive into the first-stage reactor at the same time, and the flow rates are V AC =167.1mL / hr, V H2O2 =87.0mL / hr, methanol and catalyst are mixed evenly and beating with peristaltic pump to control feeding, V MA =165.9 mL / hr. The three kettles stay for about 1.5hr, the feed flow rate per hour = 420mL / h, the stirring speed is 350rpm, and the reaction material overflows from the upper end of the reactor and enters the next reactor.
[0028] After the reaction is over, the aqueous phase catalyst slurry is separated by membrane filtration, and the catalyst filter cake or concentrated slurry is directly beate...
Embodiment 3
[0032] Single tank continuous operation, the effective volume of the tank: 500mL. Mixed slurry of methanol and catalyst, AC and H 2 o 2 Use a peristaltic pump to feed from the bottom of the reaction tank at a certain flow rate, the flow rate of the mixed slurry of catalyst and methanol is 90mL / hr, allyl chloride and 50%H 2 o 2 The flow rates are: 187mL / hr, 56mL / hr, catalyst and 50%H 2 o 2 The mass ratio of the mixture is 1:8.5, the stirring speed is 350rpm, and the reaction temperature is 41-49°C. The reaction is an exothermic reaction, and the heat is exchanged through the jacket, and the residence time is 1.5hr. The reaction material overflows from the upper end of the reactor and enters the filter device. After the water-phase catalyst slurry is separated by membrane filtration, the catalyst filter cake or concentrated slurry is directly beaten with a solvent, and then pumped into the epoxidation reaction kettle.
[0033] Reaction feeding ratio: n methanol: n chloropro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com