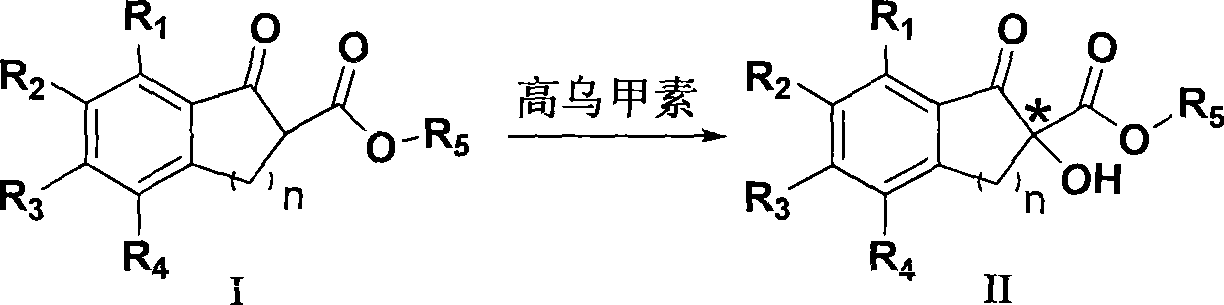
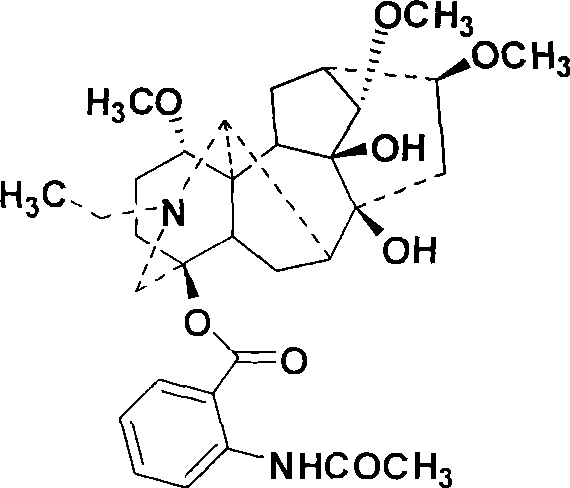
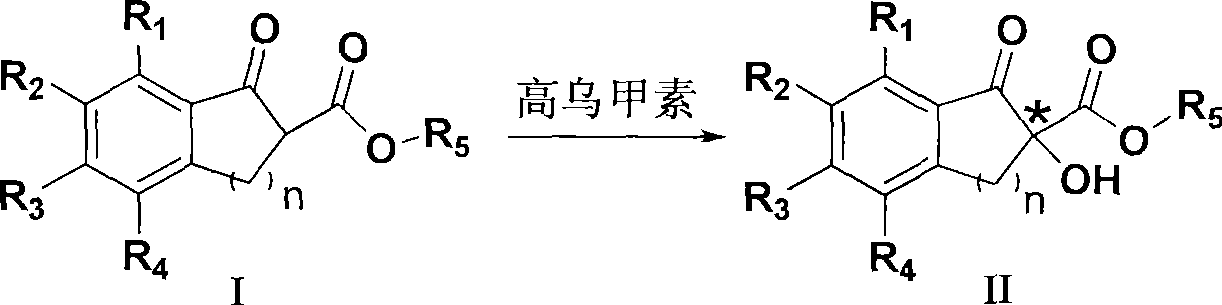
Method for preparing chiral alpha-hydroxy-beta-dicarbonyl compound with lappaconitine as catalyst
A technology of dicarbonyl compounds and gluconate, applied in the preparation of organic compounds, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem that chiral ligands and oxidants are expensive and unsuitable for production applications and operations Complicated issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of (-) 5-chloro-1-indanone-2-hydroxyl-2-carboxylic acid methyl ester
[0030] Add 2.24g (10mmol) of methyl 5-chloro-1-indanone-2-carboxylate and 0.58g (1mmol) of homomasine into 160mL of chloroform, stir and dissolve, control the temperature at 15°C, and add tert-butyl peroxide Hydrogen 4.50g (50mmol), the mixture was stirred and reacted, followed by TLC. After the reaction, the reaction solution was washed with 50 mL of 10% aqueous sodium bisulfite solution, the organic layer was separated, washed with water and brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under vacuum. The solid product was separated by silica gel column chromatography (petroleum ether / ethyl acetate=3:1) to obtain 2.21 g of white solid, yield 92%, 65% ee.
[0031] 1 H NMR (400MHz, CDCl 3 )δ 3.24(d, J=17.6Hz, 1H), 3.71(d, J=17.6Hz, 1H), 3.75(s, 3H), 4.04(bs, 1H), 7.42(d, J=8.4Hz, 1H ), 7.50 (s, 1H), 7.74 (d, J=8.4Hz, 1H); MS (API-ES Pos...
Embodiment 2
[0032] Embodiment 2: Preparation of (-) 5-chloro-1-indanone-2-hydroxyl-2-carboxylic acid methyl ester
[0033] Add 2.24g (10mmol) of methyl 5-chloro-1-indanone-2-carboxylate and 1.16g (2mmol) of chloroform into 160mL of chloroform, stir and dissolve, control the temperature at 12°C, add tert-butyl over Hydrogen oxide 4.50g (50mmol), the mixture was stirred and reacted, followed by TLC. After the reaction, the reaction solution was washed with 50 mL of 10% aqueous sodium bisulfite solution, the organic layer was separated, washed with water and brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under vacuum. The solid product was separated by silica gel column chromatography (petroleum ether / ethyl acetate=3:1) to obtain 2.23 g of white solid, yield 93%, 66% ee.
Embodiment 3
[0034] Embodiment 3: Preparation of (-) 5-chloro-1-indanone-2-hydroxyl-2-carboxylic acid methyl ester
[0035] Add 2.24g (10mmol) of methyl 5-chloro-1-indanone-2-carboxylate and 0.29g (0.5mmol) of chloroform into 160mL of chloroform, stir and dissolve, control the temperature at 15°C, add tert-butyl Hydrogen peroxide 4.50g (50mmol), the mixture was stirred and reacted, followed by TLC. After the reaction, the reaction solution was washed with 50 mL of 10% aqueous sodium bisulfite solution, the organic layer was separated, washed with water and brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under vacuum. The solid product was separated by silica gel column chromatography (petroleum ether / ethyl acetate=3:1) to obtain 2.12 g of white solid, yield 89%, 62% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com