Mutant of cyclodextrin glucosyl transferase having highly alpha-cyclodextrin yielding property and mutation method
A technology of glucosyl and cyclodextrin, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of high production cost, limited utilization, expensive commercial use, etc., and achieves the effects of being beneficial to industrialized production and improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: This example illustrates the preparation of mutant enzymes D372K, Y89D, Y89R, D372K / Y89R.
[0035] 1) Site-directed mutation
[0036] Using rapid PCR technology, single mutant enzymes D372K, Y89D and Y89R site-directed mutations: using the expression vector cgt / pET-20b(+) as a template,
[0037] The primers for site-directed mutagenesis introducing the D372K codon are:
[0038] Forward primer: 5’-GACCGGCGATGGC AAA CCCAACAACC-3' (mutated bases are underlined)
[0039] Reverse primer: 5’-GGTTGTTGGG TTT GCCATCGCCGGTC-3' (mutated base is underlined) The primer for site-directed mutagenesis to introduce the Y89D codon is:
[0040] Forward primer: 5’-CTCCGTCATCAAG GAT TCCGGCGTTA-3' (mutated bases are underlined)
[0041] Reverse primer: 5’-TAACGCCGGA ATC CTTGATGACGGAG-3' (mutated bases are underlined) to introduce the site-directed mutagenesis primer of Y89R codon:
[0042] Forward primer: 5’-CTCCGTCATCAAG CGT TCCGGCGTTA-3' (mutated bases are underlined)
[0043] R...
Embodiment 2
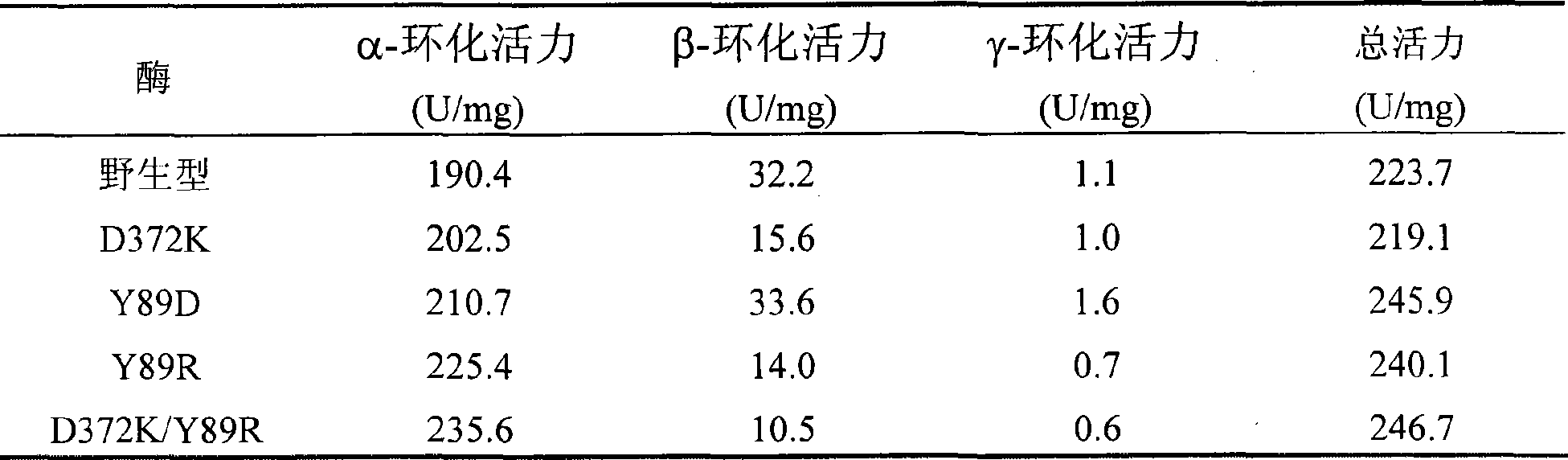
[0055] Example 2: This example illustrates enzyme activity analysis.
[0056] 1) Enzyme activity determination method:
[0057] The method of methyl orange method to determine the activity of α-cyclization: take 0.1mL of a properly diluted enzyme solution and add 0.9mL of a 3% (w / v) soluble starch solution prepared in advance with 50mM phosphate buffer (pH6.5) In, after reacting at 40°C for 10 minutes, add 1.0mL 1.0N hydrochloric acid to stop the reaction, then add 1.0mL 0.1mM methyl orange prepared with 50mM phosphate buffer, incubate at 16°C for 20min, and measure absorbance at 505nm. One unit of enzyme activity is defined as the amount of enzyme required to produce 1 μmol α-cyclodextrin per minute under this condition.
[0058] Phenolphthalein method for determination of β-cyclization activity: take 0.1 mL of appropriately diluted enzyme solution and add it to a test tube containing 0.9 mL of 3% (w / v) soluble starch solution prepared in 50 mM phosphate buffer (pH 6.5) After rea...
Embodiment 3
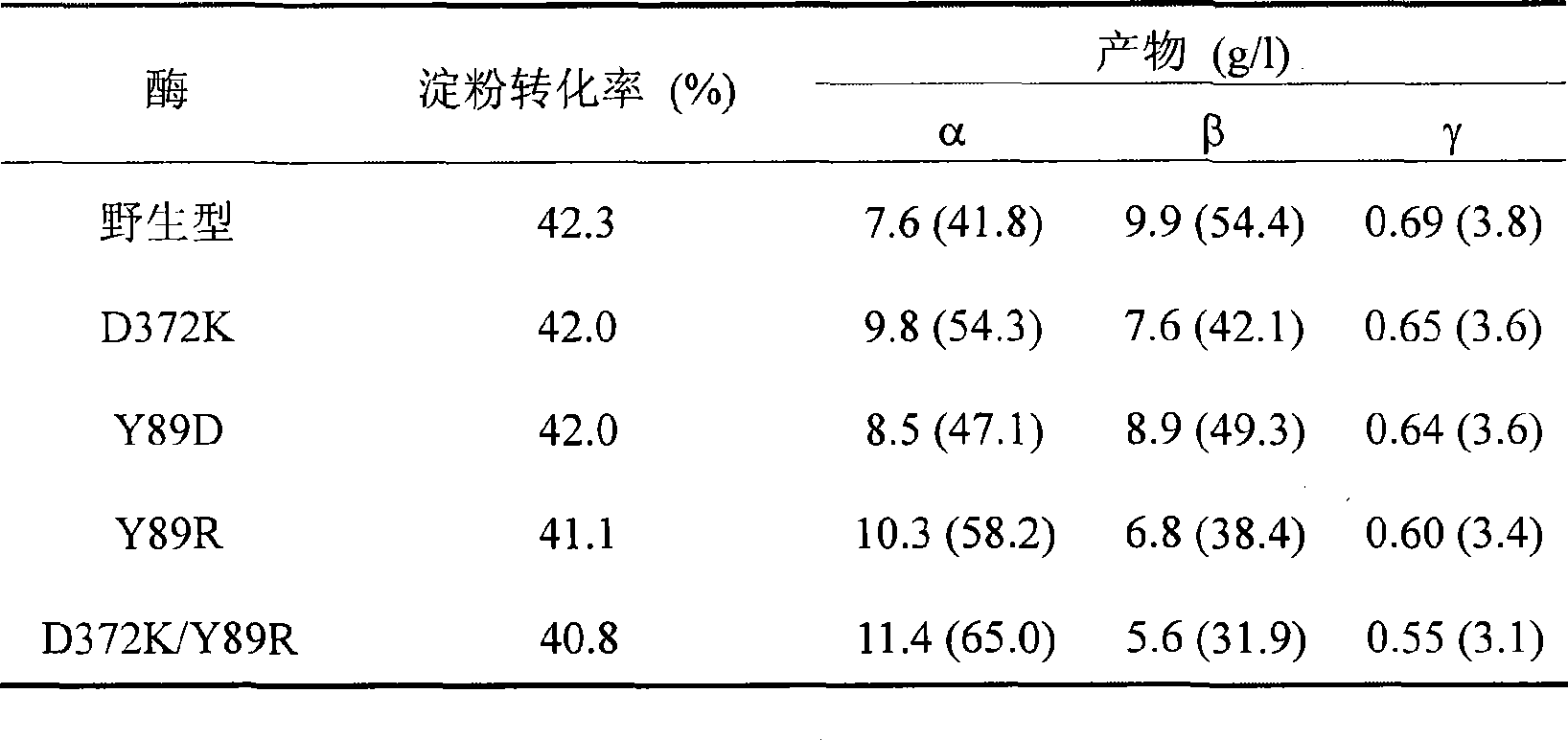
[0063] Example 3: This example illustrates the HPLC method to analyze the amount of cyclodextrin produced.
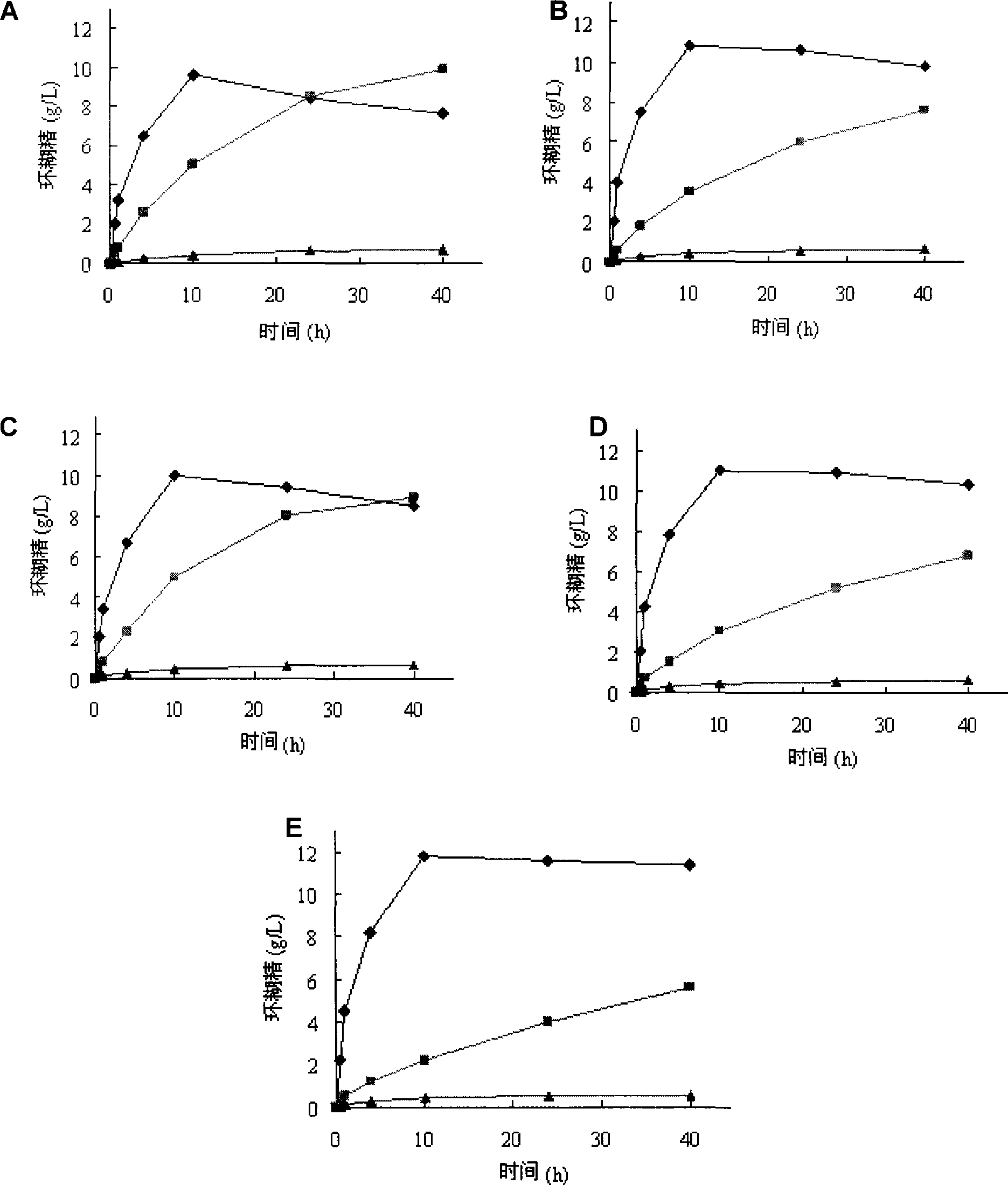
[0064] A 5% (wet base, 8% water content, w / v) soluble starch solution was prepared as a substrate, 5 g starch was dissolved in 90 mL sodium phosphate buffer (pH 6.0), the volume was adjusted to 100 mL, and the solution was boiled in boiling water for 30 minutes. Add a certain amount of wild CGT enzyme, mutant enzyme D372K, Y89D, Y89R or D372K / Y89R respectively to make the enzyme activity in the reaction system 0.2U / mL, put it at 40℃ for 40h, sample 600μL every time, centrifuge at 12000rpm for 10min, take 500μL of supernatant, add 5μL of glucoamylase (70U / mL), saccharify at 30°C for 1h, boil for 10min to inactivate, centrifuge at 12000rpm for 30min, filter the supernatant with 0.45μm ultrafiltration membrane and take 20μL for HPLC analysis.
[0065] The concentration of α-, β-, and γ-cyclodextrin in the reaction solution was determined by HPLC. The chromatographic conditions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com