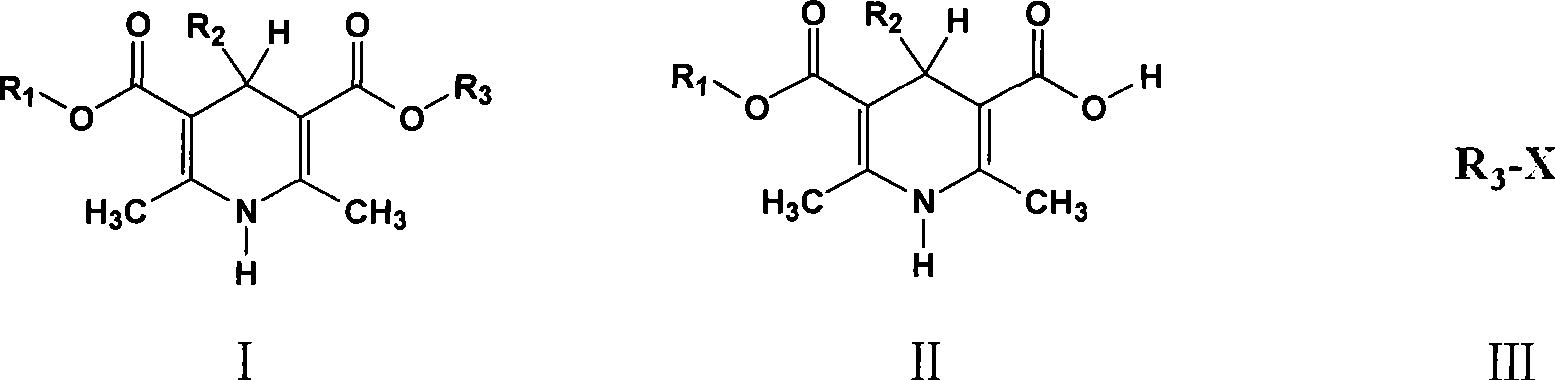
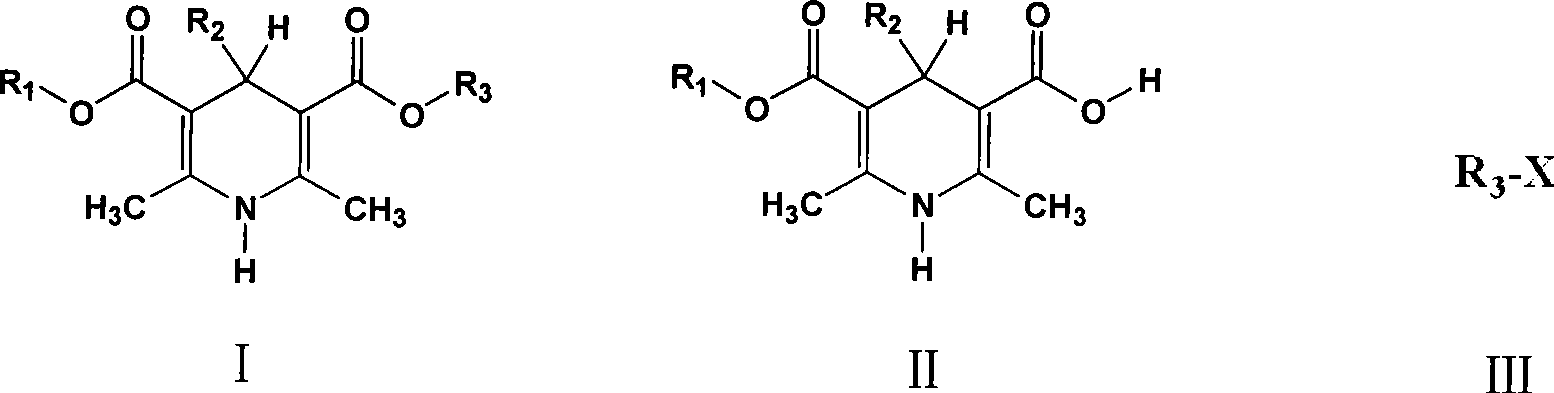
Method of preparing 1,4-dihydrogen pyridine derivatives
A technology of dihydropyridines and derivatives, applied in 1 field, can solve problems such as troublesome post-processing, serious environmental pollution, affecting product yield and quality, and achieve the effects of good product quality, high catalytic activity, and maintaining yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment one: 4-(3 / Preparation of -nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxymethoxyethyl ester isopropyl ester (compound 1)
[0017] Add 5g (14.5mmol) 4-(3 / -nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxyisopropyl-5 carboxylic acid, 1.32g (1.37ml, 17mmol,) ethylene glycol monomethyl ether, After 0.75g of D001 type cation exchange resin and 50ml of cyclohexane, heat to reflux and react until no water is carried out in the condenser tube. Cool, filter, recover the solvent, precipitate pale yellow crystals, filter and dry to obtain the crude product and recrystallize from isopropyl ether, filter, wash the crystals with isopropanol / water mixture, and suck dry. Dry under vacuum at 40°C. 4.7 g of light yellow crystalline powder (Compound 1) was obtained with a yield of 78%, a melting point of 124.5-126.5°C, a content of 99.1% as determined by HPLC, and an impurity content of 0.43%.
Embodiment 2
[0018] Embodiment two: 4-(3 / Preparation of -nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxymethoxyethyl ester isopropyl ester (compound 1)
[0019] Preparation of AlCl 3 -D001 type ion exchange resin: Soak D001 type cation exchange resin with 2mol / HCl for 24 hours, drain the acid solution, wash with distilled water until neutral, drain the water, dry at 60°C and place in a desiccator for later use. 20g of the above resin and 2g of anhydrous FeCl 3 Ethanol solution, refluxed for 8 hours, naturally cooled to room temperature, filtered, washed 3 times with distilled water, then washed 2 times with acetone, dried under vacuum at 60°C to obtain AlCl 3 -D001 type ion exchange resin, ready for use.
[0020] Add 5g (14.5mmol) 4-(3 / -nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxyisopropyl-5 carboxylic acid, 1.32g (1.37ml, 17mmol,) ethylene glycol monomethyl ether, 0.5 g of AlCl prepared above 3 After -D001 type cation exchange resin and 50ml hexanaphthene,...
Embodiment 3
[0021] Embodiment three: 4-(2 / Preparation of -nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxymethyl-2-oxopropyl ester (compound 2)
[0022] 6.6g (20mmol) 4-(2 / -Nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxymethyl-5-carboxylic acid, 0.55g 717 type anion exchange resin and 50ml DMF were stirred and mixed, then added 2.75g (23mmol) Acetyl methyl nitrate was reacted at 65°C for 4 hours, filtered, cooled, and pale yellow crystals were precipitated, filtered, and dried to obtain a crude product, which was recrystallized in 3:7 ethanol water, filtered, and crystallized with cooled ethanol / Water (3:7 V / V, volume ratio) mixture was washed and drained. Dry under vacuum at 40°C. 7.1 g of light yellow crystalline powder (compound 2) was obtained with a yield of 91.5%, a melting point of 149.3-152.6°C, a content of 98.7% as determined by HPLC, and an impurity content of 0.98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com